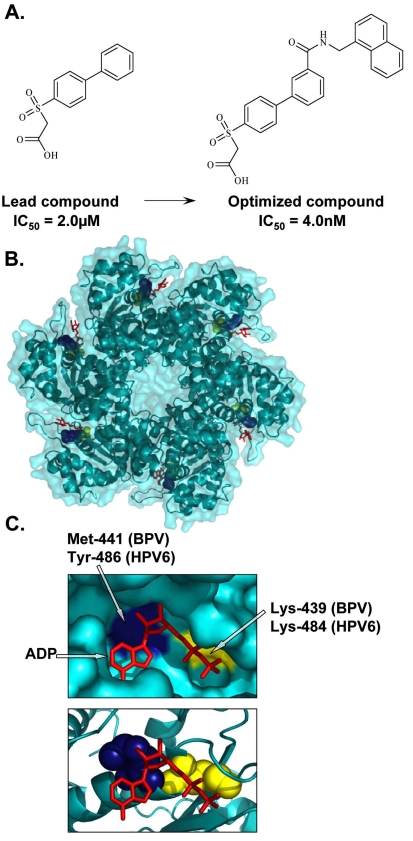
Fig. (3).
Inhibition of E1 ATPase activity. (A) Structures of the lead and optimized biphenylsulfonacetic acid inhibitors of the E1 ATPase activity with IC50 values of 2.0µM and 4.0nM, respectively. (B) Crystal structure of the hexameric C-terminal helicase domain of bovine papillomavirus (BPV) E1 (PDB accession number 2GXA [173]). (C) Enlarged views of the ATP-binding pocket displaying the locations of the highly conserved catalytic Lys-439 (yellow), essential for ATP interaction and catalysis, and Met-441, (blue) important for the activity of biphenylsulfonacetic acid inhibitors. These residues are equivalent to Lys-484 and Tyr-486, respectively, in HPV6 E1. Bound ADP (red) is depicted in stick representation.