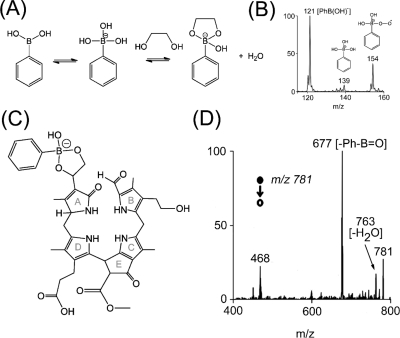
Figure 3.
(A) Rapid and reversible covalent complexation of phenylboronic acid with 1,2-diols to form cyclic boronates in basic aqueous media.(53) (B) Reactive species generated in solution and detected during reactive DESI-MS. (C) Proposed structure of the cyclization product of phenylboronic acid and the chlorophyll catabolite. (D) Negative ion mode reactive DESI-MS/MS spectrum of a hophornbeam (O. virginiana) leaf extract spotted on a PTFE surface. Spray solvent at a flow rate of 3 μL/min was 3 mM phenylboronic acid reduced to pH 9 using aqueous ammonia. The deprotonated cyclization product of phenylboronic acid and the chlorophyll catabolite at m/z 781 showed characteristic loss of Ph-B=O.