Abstract
Background
The practice of coiling APCs before Fontan completion is controversial and published data are limited. We sought to compare outcomes in subjects with and without pre-Fontan coil embolization of aortopulmonary collaterals (APCs) using the Pediatric Heart Network (PHN) Fontan Cross-Sectional Study database which enrolled survivors of prior Fontan palliation.
Methods
We compared hospital length of stay (LOS) after Fontan in 80 subjects who underwent APC coiling with 459 subjects who did not. Secondary outcomes included post-Fontan complications and assessment of health status and ventricular performance at cross-sectional evaluation (mean 8.6±3.4 years after Fontan).
Results
Centers varied markedly in frequency of pre-Fontan APC coiling (range 0-30% of subjects, p<0.001). The coil group was older at Fontan (p=0.004), and more likely to have single right ventricular morphology (p=0.054) and pre-Fontan atrioventricular valve regurgitation (p=0.03). The coil group underwent Fontan surgery more recently (p<0.001), was more likely to have a prior superior cavopulmonary anastomosis (p<0.001), and more likely to undergo extracardiac Fontan connection (p<0.001) and surgical fenestration (p<0.001). In multivariable analyses, APC coiling was not associated with LOS (hazard ratio (HR) for remaining in-hospital 0.91, 95% CI 0.70-1.18, p=0.48) or postoperative complications, except more post-Fontan catheter interventions (HR 1.74, 95% CI 1.04-2.91, p=0.03), primarily additional APC coils. The groups had similar outcomes at cross-sectional evaluation.
Conclusion
Management of APCs before Fontan shows marked practice variation. We did not find an association between pre-Fontan coiling of APCs and shorter post-operative hospital stay or with better late outcomes. Prospective studies of this practice are needed.
Aortopulmonary collaterals (APCs) are common in single ventricle patients,1 but their hemodynamic importance has been a matter of debate. Some reports indicate that patients who undergo the Fontan procedure with APCs have greater perioperative mortality2, 3 and morbidity, including longer duration of inotropic support, pleural drainage, ventilation and hospital stay.1, 2 The presence of APCs has also been correlated with longer-term mortality and morbidity, such as heart failure.3 Moreover, some studies have indicated that pre-Fontan coil occlusion of APCs reduces complications such as duration of pleural drainage.4 However, other studies have found similar outcomes in those with and without significant APC flow.4-6
Determining the benefits of coil embolization of APCs prior to Fontan surgery is becoming increasingly important because non-invasive imaging modalities may replace routine pre-Fontan cardiac catheterization in some patients.7, 8 Cardiac catheterization in pediatric patients is associated with complication rates ranging from 7-25%.9-12 Furthermore, ionizing radiation exposure in childhood is associated with an increased risk of cancer13, 14 and interventional procedures such as APC coiling increase both fluoroscopy time and cost of the procedure. Therefore, there has been recent interest in reassessing the benefits of pre-Fontan catheterization including interventional procedures such as APC coiling.17-19
Retrospectively evaluating the effects of pre-operative APC coiling is challenging, however, because the indications for coiling are ill-defined and vary by center, and quantification methods for collateral blood flow have not been standardized. Furthermore, almost all previous studies drew inferences from small samples, and all are derived from single center experiences. No randomized controlled trials have evaluated the effect of pre-Fontan APC embolization. Thus, differences in reported outcomes of APC coiling could reflect variation in patient and center characteristics rather than the actual effects of the APC coiling.
The Pediatric Heart Network’s (PHN) Fontan Cross-Sectional Study characterized a large cohort of survivors who had previously undergone the Fontan procedure, with assessment of functional health status, ventricular size and function, exercise capacity, and brain natriuretic peptide (BNP). Using the database of this multi-center study, we sought to describe practice variability surrounding management of APCs prior to Fontan completion, and to determine whether outcomes after the Fontan procedure were improved by pre-Fontan coil embolization of APCs.
Methods
Study Subjects
From March 2003 through April 2004, the seven centers comprising the PHN performed a multi-center, cross-sectional assessment of survivors of prior Fontan palliation. The techniques used to collect medical history data and to perform standardized echocardiography, cardiac magnetic resonance imaging (CMR), exercise testing, and measurement of BNP have been previously described.15 Potential study subjects were identified from medical record review, and deemed eligible if they were ages 6 to 18 years at the time of enrollment; had undergone Fontan procedure at least 6 months before initial study testing; and agreed to have an echocardiogram and blood testing and to complete a parent report functional health status questionnaire within 3 months of enrollment at one of the study centers. Exclusion criteria included the presence of a non-cardiac medical or psychiatric disorder that would prevent successful completion or invalidate the results of study testing. For the present analyses, we also excluded subjects who had undergone a Fontan conversion operation, because the database did not include information about peri-operative course during their initial Fontan. The protocol was approved by each center’s institutional review board and written informed consent and assent were obtained. Subjects who had undergone APC coiling at any catheterization prior to Fontan completion were classified as the “coil group”.
Measurements
Variables chosen for the current analyses included demographics, ventricular morphology, prior surgical procedures, and pre-Fontan clinical status including echocardiographic and catheterization assessments and interventional procedures. We also examined Fontan surgical data, including year of Fontan, procedural variables (e.g., presence of fenestration and type of Fontan connection), and postoperative short- and long-term outcomes including reinterventions. We chose hospital length of stay after the Fontan procedure as our primary perioperative outcome variable. Additional variables selected were post-Fontan complications and interventions as well as results of testing at cross-sectional assessment including ejection fraction by echocardiography using the long plus short axis biplane modified Simpson’s method, percent predicted maximal oxygen consumption and the percent predicted ventilatory anaerobic threshold on cycle ergometry exercise testing, both normalized for age and gender, and functional health status and neurodevelopmental outcomes assessed by the Child Health Questionnaire – Parent Form 50 (CHQ-PF50), a measure of functional health status and general well-being.16
Statistical Analyses
Summary statistics are presented as mean ± standard deviation, median and interquartile range, or percentages. Cox proportional hazards modeling was used to compare the distributions of time to discharge from the Fontan hospitalization by APC coil status. Multivariable modeling of Fontan length of stay was conducted to obtain the covariate-adjusted hazard ratio for remaining in-hospital. Seven variables were chosen as covariates for potential inclusion in the multivariate model using stepwise regression if the p-value was < 0.05: gender, dominant ventricular type (right vs. left vs. mixed), Stage II surgery performed (yes vs. no), presence vs. absence of a surgical fenestration, type of Fontan connection, age at Fontan, and era of Fontan procedure (1986-1991 vs. 1992-1997 vs. 1998-2002). Length of stay (LOS) was also compared by APC coil status utilizing these univariate and covariate-adjusted analyses, in an analysis cohort restricted to subjects with a prior superior cavopulmonary anastomosis.
Secondary outcomes by APC coil status were modeled similarly using linear regression for exercise performance measures, log BNP, and ejection fraction at the time of cross-sectional assessment, and logistic regression for the presence vs. absence of clinical complications such as a history of protein losing enteropathy (PLE), stroke, thrombosis, or pleural effusions. For secondary outcomes, 7 variables were considered for inclusion as adjustment factors: gender, ventricular type (dominance), Stage II surgery performed (yes vs. no), time since Fontan, type of Fontan procedure, age at Fontan, and era of Fontan procedure (1986-1991 vs. 1992-1997 vs. 1998-2002). Comparisons of exercise performance measures were restricted to the subset of subjects who achieved maximal effort, defined as a respiratory exchange ratio of ≥ 1.1. To determine whether APC coiling was associated with outcomes for selected subgroups of subjects, we performed tests of interaction between APC coiling and two subgroup factors, defined both continuously and by rounded quartile: pre-Fontan oxygen saturation and age at Fontan procedure.
Results
The Fontan Cross-Sectional Study database included 546 subjects, of whom 7 were excluded because their index surgery was a Fontan conversion. Mean age at Fontan was 3.4±2.1 years with a mean age at enrollment of 11.9±3.4 years and a mean time since Fontan of 8.6±3.4 years. Of the 539 included subjects, 80 (15%) had undergone coiling of APCs prior to the Fontan (“coil group”) and 459 had not (“no coil group”). Of the 7 excluded subjects, 1 had undergone APC coiling and 6 had not.
The percentage of subjects undergoing pre-Fontan coiling varied significantly by center (Figure 1), with a range of 0-30% (p<0.001). There was no association between the percentage of subjects undergoing coiling and center enrollment volume: the center with the lowest percentage of subjects who underwent coil occlusion (0%) enrolled 102 subjects in the study while the center with the highest percentage (30%) enrolled 103.
Figure 1. Frequency of Coil Embolization of Aortopulmonary Collateral Vessels Among Centers.

Center-specific proportions of subjects with aortopulmonary collateral coils (p<0.001). Each bar represents one of the 7 centers in decreasing order of center sample size, with the number of subjects enrolled from the center listed atop the bar.
Compared to subjects who never received APC coils (Table 1), the coil group was older at Fontan and was more likely to have a single right ventricle. At pre-Fontan testing, the coil group was also more likely to have moderate or severe atrioventricular valve regurgitation by pre-Fontan echocardiography, and slightly higher systemic oxygen saturation at pre-Fontan catheterization. Furthermore, those with pre-Fontan APC coils were more likely to have undergone additional catheter interventions to occlude veno-venous collaterals. Pre-Fontan characteristics were otherwise similar between the two groups, including weight-for age, degree of pre-Fontan ventricular dysfunction on echocardiography, ventricular end-diastolic pressure, and presence of pulmonary artery stenosis or superior vena cava obstruction at catheterization.
Table 1.
Subject Characteristics at Fontan Completion According to APC Coil Status
| Coil Group | No Coil Group | p-value | |
|---|---|---|---|
| Subject Demographics | |||
| Age at Fontan, yr (mean±SD) | 3.9±2.2 | 3.2±1.9 | 0.004 |
| Ventricular type | 0.05 | ||
| Left | 37% | 50% | |
| Right | 45% | 32% | |
| Mixed | 18% | 18% | |
|
| |||
| Pre-Fontan Echocardiographic Findings | |||
| Atrioventricular valve regurgitation | |||
| Any | 62% | 48% | 0.03 |
| Moderate or severe | 13% | 4% | 0.005 |
|
| |||
| Pre-Fontan Catheterization Findings | |||
| Systemic oxygen saturation (mean±SD) | 85±4% | 84±5% | 0.02 |
| Veno-venous collateral coils | 18% | 7% | 0.003 |
|
| |||
| Fontan Surgical Characteristics | |||
| Year of Fontan | <0.001 | ||
| 1986-1991 | 5% | 24% | |
| 1992-1997 | 40% | 56% | |
| 1998-2002 | 55% | 21% | |
| Prior superior cavopulmonary anastomosis | 98% | 71% | <0.001 |
| Type of Fontan | <0.001 | ||
| Atriopulmonary connection | 0% | 16% | |
| Intracardiac lateral tunnel | 59% | 60% | |
| Extracardiac lateral tunnel | 20% | 11% | |
| Extracardiac conduit | 21% | 11% | |
| Other | 0% | 2% | |
| Surgical Fenestration | 84% | 65% | <0.001 |
As shown in Table 1, subjects in the coil group underwent Fontan completion in a more recent calendar year and were more likely to have had a prior superior cavopulmonary anastomosis. Fontan surgery in the coil group, compared to the no-coil group, was more likely to include a surgical fenestration, and the Fontan connection itself was more likely to be an extracardiac Fontan and less likely to involve an atriopulmonary connection. Cardiopulmonary bypass times were similar in the two groups (p=0.99).
In univariable analysis, length of stay after Fontan was shorter for subjects in the coil group (median 10.5 days vs. 12.0 days, p=0.03). The coil group also had a greater median number of interventions at cardiac catheterizations after the Fontan procedure (1 vs. 0, p=0.04). Exercise test results, ejection fraction and general health status, as reflected in the Physical Summary score of the CHQ-PF50, were similar in the groups at the time of cross-sectional testing. No other post-Fontan outcome variable or in-person test result differed significantly between those with and without pre-Fontan APC coils. There was no correlation between time from coil placement to Fontan completion and length of stay after Fontan (Spearman R = 0.015, p=0.9)
In multivariable regression, adjusting for year of Fontan and surgical fenestration, the two groups were similar in their post-Fontan hospital length of stay (Figure 2). To further reduce the risk of residual confounding, we restricted the analyses to subjects who underwent a superior cavopulmonary anastomosis prior to Fontan completion (75% of the sample). Even in this subgroup, LOS did not differ significantly between the coil (n=78) versus no-coil (n=327) groups in either unadjusted (median 11 days for both groups, p=0.28) or multivariable analyses (hazard ratio for remaining in the hospital 0.90, 95% CI 0.70-1.16, p=0.43). Furthermore, length of stay was also similar between subjects from the two centers with the highest and lowest frequency of APC coils (median 9.5 vs. 10 days, respectively), both large tertiary care centers enrolling a similar number of subjects.
Figure 2. Post-Fontan Hospital Length of Stay After Adjustment for Fenestration and Year of Fontan.
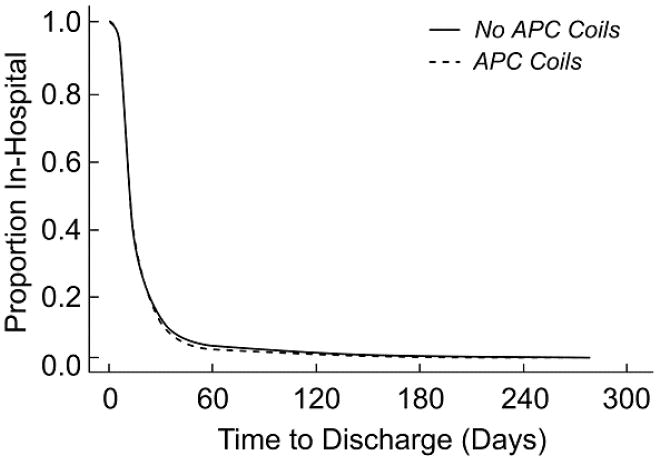
Proportion of subjects in the hospital vs. days after Fontan for each group, after adjusting for surgical fenestration and year of Fontan. Hazard ratio for remaining in the hospital for subjects in the coil vs. no coil groups is 0.91, 95% CI 0.70-1.18, p =0.48. Abbreviation: APC – aortopulmonary collateral.
Among the secondary outcomes (Table 2), even after adjusting for type of Fontan connection and years since Fontan completion, the coil group was more likely to have had one or more post-Fontan catheter interventions (odds ratio (OR) 1.74, 95% CI 1.04-2.91, p=0.03), consisting primarily of additional APC coils (23% vs. 13% of subjects with post-Fontan catheter interventions in the coil vs. no-coil group, respectively). The groups did not, however, differ in any other outcomes, including occurrence of pleural effusions, late complications following Fontan completion and test results at cross-sectional follow-up. There were also no significant differences in frequency of neurodevelopmental problems reported on the CHQ-PF50 including developmental delay (p=0.88), learning problems (p=0.47), attention problems (p=0.82), speech problems (p=0.53), or behavioral problems (p=0.92).
Table 2.
Post-Fontan Outcomes for the Coil vs. the No-Coil Group After Adjustment for Covariates*
| Surgical Outcomes | Covariate-adjusted Hazard or Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Fontan Length of Stay | 0.91 | 0.70-1.18 | 0.48 |
| Pleural Effusion | 1.00 | 0.21-4.65 | 1.00 |
|
| |||
| Late complications | Covariate-adjusted Hazard Ratio | 95% CI | p-value |
| Catheterization Interventions | 1.74 | 1.04-2.91 | 0.03 |
| Cardiac Surgeries | 1.03 | 0.53-1.97 | 0.94 |
| Protein Losing Enteropathy | 0.91 | 0.19-4.27 | 0.90 |
| Strokes | 1.00 | 0.12-8.51 | 1.00 |
| Seizures | 0.47 | 0.06-3.81 | 0.48 |
| Thrombosis | 1.48 | 0.58-3.76 | 0.41 |
|
| |||
| Testing at Cross-sectional Follow-up | Coils Adjusted Mean ± SE | No Coils Adjusted Mean ± SE | p-value |
| Ejection Fraction, % | 59.3±1.33 | 58.7±0.70 | 0.66 |
| Exercise test results | |||
| Percent predicted peak VO2, % | 69.0±3.1 | 66.5±1.2 | 0.45 |
| Percent predicted VAT, % | 72.1±4.6 | 77.8±1.9 | 0.25 |
| Log (BNP level, pg/mL) | 2.85±0.13 | 2.80±0.07 | 0.71 |
| Child Health Questionnaire | |||
| Physical Summary Score | 44.8±1.4 | 45.4±0.6 | 0.66 |
| Psychosocial Summary Score | 45.4±1.2 | 47.6±0.5 | 0.10 |
Covariates considered for inclusion in the final model: year of Fontan, gender, ventricular type, prior superior cavopulmonary anastomosis, type of Fontan connection, age at Fontan, presence of surgical fenestration (for post-operative outcomes), and time since Fontan (for outcomes at follow-up and testing). Abbreviations: VO2 – oxygen consumption; VAT – ventilatory anaerobic threshold; BNP – brain natriuretic peptide.
We explored whether coils were more effective in subgroups of subjects expected to have more APC flow because of their oxygen saturation or age at Fontan. We found no subject subset, defined by oxygen saturation or by age at Fontan, for whom APC coiling was significantly associated with shorter length of stay after the Fontan procedure. With regard to other outcomes, including those measured at cross-sectional follow-up, only one interaction reached statistical significance. An association between ejection fraction and APC coiling depended on age at Fontan (p=0.004); among those with Fontan performed at older ages (4 years or older), ejection fraction was lower in the coil group compared with the no coil group (55±13% vs. 59±11%, respectively).
Discussion
Single ventricle patients frequently develop abnormal vascular connections from the systemic to the pulmonary circulation.1 In this setting, APCs can present a hemodynamic burden by increasing pulmonary venous return to the systemic ventricle, thereby increasing volume overload. Furthermore, blood flow returning from APCs through the pulmonary veins during cardiopulmonary bypass can obscure visualization of the surgical field and may reduce cerebral protection at the time of Fontan surgery. After Fontan completion, the potential for a competing source of pulmonary blood flow at relatively high pressures, and the associated increase in end diastolic pressure of the single ventricle, may result in diminished Fontan flow and increased systemic venous pressures. This, in turn, may increase early postoperative morbidity, such as prolonged post-operative pleural drainage, as well as long-term morbidities such as development of PLE or Fontan failure. Occlusion of such APCs, therefore, would be expected to provide a clinical benefit in affected patients. Despite these considerations, the efficacy of coil occluding APCs prior to the Fontan procedure remains under debate.
We found that PHN centers varied widely in the frequency with which APCs were coil occluded prior to the Fontan procedure in subjects with univentricular hearts. Furthermore, subjects who underwent APC coiling prior to Fontan were more likely to undergo additional APC coiling after Fontan, likely reflecting practice variations among sites. Although univariable analyses suggested shorter hospital length of stay after the Fontan procedure in subjects who underwent APC coiling, multivariable analysis did not demonstrate an association between pre-Fontan coiling of APCs and faster postoperative recovery. Furthermore, we could not find beneficial effects of coil embolization on post-operative morbidity or late outcomes, including echocardiographic ejection fraction, exercise tolerance, functional health status, or frequency of neurodevelopmental problems.
Prior studies evaluating the hemodynamic importance of APCs around the time of the Fontan operation show conflicting data. Ichikawa and colleagues measured collateral flow in 33 subjects as a percentage of total flow on cardiopulmonary bypass during Fontan surgery.17 They found that higher APC flow was associated with greater post-operative systemic venous pressures and mortality. Their sample size was small, however, and they did not adjust for potential confounders such as pulmonary artery stenosis, ventricular dysfunction or atrioventricular valve regurgitation. Furthermore, only one of their subjects had had a prior superior cavopulmonary anastomosis, most (n=20) had undergone atriopulmonary Fontan connections, and none of the procedures included a surgical fenestration. In addition to institutional preferences, these differences in practice likely also reflect the era in which their subjects underwent Fontan completion (1987-1990). With a more recent cohort (1997-2000), Bradley and colleagues4 found that in 32 subjects undergoing Fontan, all with prior superior cavopulmonary anastomosis and either a lateral tunnel or extracardiac fenestrated Fontan, APC flow was not associated with post-operative hemodynamics, resource utilization or early outcomes.
Coil occlusion of APCs is also controversial. Spicer and colleagues retrospectively reviewed 71 subjects who underwent Fontan completion at a single institution and visually graded APC flow at catheterization.2 They found that high APC flow at catheterization prior to Fontan was associated with prolonged pleural drainage after Fontan. Among those with high APC flow (n=30), coil (n=11) or surgical (n=2) occlusion of these vessels was associated with shorter post-operative pleural drainage. Conversely, McElhinney and colleagues reported that coil embolization of APCs in 14 out of 22 subjects with high collateral flow on pre-Fontan catheterization was not associated with postoperative outcomes.15 Neither of these studies was designed to evaluate the effect of APC coil occlusion, however, and the sample of subjects with high collateral flow who underwent coil embolization in each was small, limiting inferences regarding the effects of APC coiling. With a large cohort of subjects from multiple centers, we found no association between coil embolization of APCs and post-Fontan hospital length of stay, a surrogate for duration of pleural drainage. Length of stay was also similar between the two large tertiary care centers with similar subject enrollment numbers and the highest and lowest frequency of pre-Fontan APC coils.
Our data should be viewed in light of certain limitations. First, medical history prior to the cross-sectional evaluation was ascertained by retrospective review. Second, because the Fontan Cross-Sectional Study evaluated only transplant-free survivors of the Fontan procedure, our study could not explore whether pre-Fontan APC coiling was associated with mortality or likelihood of cardiac transplantation after the Fontan procedure. Furthermore, exclusion from our analyses of the 7 subjects who underwent Fontan conversions could have resulted in underestimation of the effects of APC coiling on Fontan failure; however, the number of such subjects was small, and the proportion with and without APC coils was similar to that in the overall study group. It is also possible that unrecorded variations in management such as in aspects of intra-operative management during the Fontan procedure could have influenced the immediate post-operative course and confounded our results. Although unlikely, we cannot exclude the possibility that differences between the coil groups might emerge at longer duration of follow-up. Finally, small numbers of subjects within subgroups limited our power to detect interactions.
Perhaps the most important limitation of our analysis is the absence of information in the database on the amount of APC flow. It is possible that a subset of subjects with large, discrete APCs would benefit from pre-Fontan coiling. However, if many subjects underwent coiling of only small APCs that carry a relatively small proportion of pulmonary blood flow, the benefits of coil embolization of larger APCs could have been obscured. It is also possible that, in subjects with innumerable small APCs, coil occlusion removed only a small proportion of excess pulmonary blood flow, attenuating the differences in outcomes between the coil and no coil groups. Recently described CMR techniques to quantify collateral flow before and after coil occlusion may help answer these questions and assist with the identification of patients who may benefit from coil embolization.18, 19 Since the PHN Fontan database predated widespread use of CMR quantification of APC flow, this information was not available for our cohort and detection of collaterals by conventional angiography can be variable and unreliable.20 Of note, subjects at the two centers with the most disparate practice management surrounding APCs had similar post-Fontan LOS. Because these centers had similar patient volume and complexity, differences in their frequency of coil embolization are likely to represent practice variation rather than differences in subject characteristics such as collateral load. Coil embolization thus might be unnecessary in a substantial number of single ventricle patients who currently undergo this procedure.
Despite its limitations, our secondary analysis of a large, multicenter database of Fontan survivors showed that preoperative coil occlusion of APCs is subject to wide practice variability and, in our cohort of survivors of prior Fontan palliation, was not associated with reduced Fontan length of stay or better late Fontan outcomes. The only treatment group difference was that subjects with pre-Fontan APC coils had a higher number of pre-Fontan veno-venous collateral coiling and a higher rate of post-Fontan cardiac catheterization interventions, primarily additional APC coils, likely reflecting the practice variation between centers. Placement of APC coils entails additional ionizing radiation in young children, extends hospital stay after catheterization, and increases financial expense. Thus, prospective studies are needed to better delineate the efficacy of APC coiling in patients with univentricular heart and to clarify which patients have the highest likelihood of benefit.
Acknowledgments
Funding Sources: This publication was made possible by Grant Numbers HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288 from the National Heart Lung and Blood Institute. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or NIH.
Abbreviations
- APC
aortopulmonary collaterals
- BNP
brain natriuretic peptide
- CHQ-PF50
Child Health Questionnaire – Parent Form 50
- LOS
length of stay
- PHN
Pediatric Heart Network
- PLE
protein losing enteropathy
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanter KR, Vincent RN. Management of aortopulmonary collateral arteries in Fontan patients: occlusion improves clinical outcome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:48–54. doi: 10.1053/pcsu.2002.31501. [DOI] [PubMed] [Google Scholar]
- 2.Spicer RL, Uzark KC, Moore JW, et al. Aortopulmonary collateral vessels and prolonged pleural effusions after modified Fontan procedures. Am Heart J. 1996;131:1164–8. doi: 10.1016/s0002-8703(96)90092-7. [DOI] [PubMed] [Google Scholar]
- 3.Kanter KR, Vincent RN, Raviele AA. Importance of acquired systemic-to-pulmonary collaterals in the Fontan operation. Ann Thorac Surg. 1999;68:969–974. doi: 10.1016/s0003-4975(99)00782-1. [DOI] [PubMed] [Google Scholar]
- 4.Bradley SM, McCall MM, Sistino JJ, et al. Aortopulmonary collateral flow in the Fontan patient: does it matter? Ann Thorac Surg. 2001;72:408–15. doi: 10.1016/s0003-4975(01)02813-2. [DOI] [PubMed] [Google Scholar]
- 5.Lim DS, Graziano JN, Rocchini AP, et al. Transcatheter occlusion of aortopulmonary shunts during single-ventricle surgical palliation. Catheter Cardiovasc Interv. 2005;65:427–33. doi: 10.1002/ccd.20403. [DOI] [PubMed] [Google Scholar]
- 6.McElhinney DB, Reddy VM, Tworetzky W, et al. Incidence and implications of systemic to pulmonary collaterals after bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2000;69:1222–8. doi: 10.1016/s0003-4975(99)01088-7. [DOI] [PubMed] [Google Scholar]
- 7.Brown DW, Gauvreau K, Powell AJ, et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis in infants with functional single ventricle: a prospective randomized trial. Circulation. 2007;116:2718–25. doi: 10.1161/CIRCULATIONAHA.107.723213. [DOI] [PubMed] [Google Scholar]
- 8.Fogel MA. Is routine cardiac catheterization necessary in the management of patients with single ventricles across staged Fontan reconstruction? No! Pediatr Cardiol. 2005;26:154–8. doi: 10.1007/s00246-004-0960-6. [DOI] [PubMed] [Google Scholar]
- 9.Brown DW, Gauvreau K, Moran AM, et al. Clinical outcomes and utility of cardiac catheterization prior to superior cavopulmonary anastomosis. J Thorac Cardiovasc Surg. 2003;126:272–81. doi: 10.1016/s0022-5223(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 10.Mehta R, Lee K-J, Chaturvedi R, et al. Complications of pediatric cardiac catheterization: A review in the current era. Catheter Cardiovasc Interv. 2008;72:278–285. doi: 10.1002/ccd.21580. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BL, Cabalka AK, Hagler DJ, et al. Procedural complications during congenital cardiac catheterization. Congenit Heart Dis. 2011;5:118–23. doi: 10.1111/j.1747-0803.2010.00385.x. [DOI] [PubMed] [Google Scholar]
- 12.Vitiello R, McCrindle BW, Nykanen D, et al. Complications associated with pediatric cardiac catheterization. J Am Coll Cardiol. 1998;32:1433–40. doi: 10.1016/s0735-1097(98)00396-9. [DOI] [PubMed] [Google Scholar]
- 13.Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Pediatr Radiol. 2002;32:700–6. doi: 10.1007/s00247-002-0774-8. [DOI] [PubMed] [Google Scholar]
- 14.Boothroyd A, McDonald E, Moores BM, et al. Radiation exposure to children during cardiac catheterization. Br J Radiol. 1997;70:180–5. doi: 10.1259/bjr.70.830.9135445. [DOI] [PubMed] [Google Scholar]
- 15.Sleeper LA, Anderson PT, Hsu D, et al. Design of a large cross-sectional study to facilitate future clinical trials in children with the Fontan palliation. Am Heart J. 2006;152:427–433. doi: 10.1016/j.ahj.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landgraf JM, Abetz LN, Ware JE. The Child Health Questionnaire (CHQ) User’s Manual. Boston: The Health Institute, New England Medical Center; 1999. [Google Scholar]
- 17.Ichikawa H, Yagihara T, Kishimoto H, et al. Extent of aortopulmonary collateral blood flow as a risk factor for Fontan operations. Ann Thorac Surg. 1995;59:433–437. doi: 10.1016/0003-4975(94)00120-v. [DOI] [PubMed] [Google Scholar]
- 18.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging. 2009;2:219–25. doi: 10.1161/CIRCIMAGING.108.834192. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead KK, Gillespie MJ, Harris MA, et al. Noninvasive quantification of systemic-to-pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging. 2009;2:405–11. doi: 10.1161/CIRCIMAGING.108.832113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triedman JK, Bridges ND, Mayer JE, Jr, et al. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22:207–15. doi: 10.1016/0735-1097(93)90836-p. [DOI] [PubMed] [Google Scholar]


