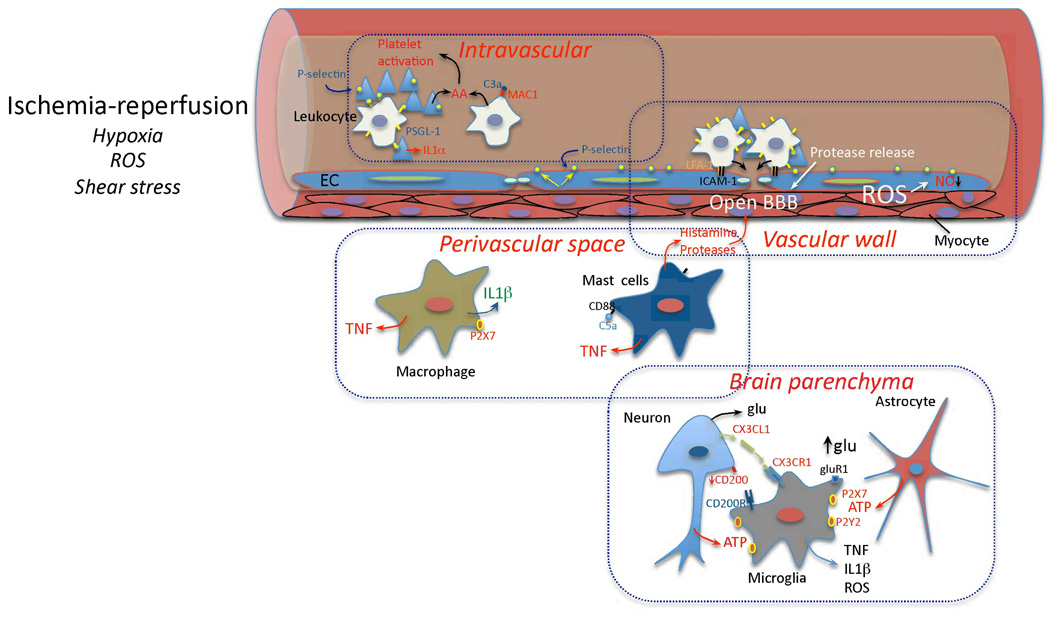
Figure 1. Early vascular, perivascular and parenchymal events triggered by I/R.
Hypoxia, ROS and changes in shear stress initiate the cellular events induced by I/R. In the vessels lumen, I/R leads to blood clotting, platelet aggregation and cytokine release (IL-1α). Translocation of P-selectin on the surface of platelets and EC leads to platelet-leukocyte aggregation. Complement is activated and arachidonic acid metabolites are released. In the vascular wall, upregulation of E- and P-selectin on EC provides a platform for low affinity leukocyte binding through interaction with sialyl Lex moieties of glycoproteins expressed on leukocytes, e.g. PSGL-1. Firm adhesion is obtained after endothelial expression of ICAM-1 interacting with leukocyte β2 integrins (LFA-1 and Mac-1)144. Loss of NO promotes vasoconstriction and enhances leukocyte and platelet aggregation. MMP activation could lead to BBB breakdown and matrix proteolysis facilitating leukocyte extravasation. In the perivascular space, chemotactic complement subunits (C5a) acting on mast cell complement receptors (CD88) leads to degranulation and release of histamine and proteases, contributing to BBB leakiness. Cytokines (TNF, IL-1β) are produced by mast cells and perivascular macrophages, providing further signals to guide leukocyte migration across the vessel wall145,146. In the brain parenchyma, injured cells release purines (ATP), which act as early proinflammatory signals leading to production of cytokines and chemokines. Disruption of neuronal-microglial interaction (CX3CL1, CD200) and increases in extracellular glutamate (glu) acting on microglial GluR1 metabotropic receptor27 also contribute to the pro-inflammatory milieu.