Abstract
BACKGROUND
Carriers of a premutation (CGG repeat length 55–200) in the fragile X mental retardation (FMR1) gene are at risk for primary ovarian insufficiency (FXPOI). The anti-Müllerian hormone (AMH) level acts as a useful marker of ovarian follicle reserve and, thus, may serve to predict when this ovarian reserve becomes too low to sustain ovarian function. We investigated the intra-individual variation of AMH levels over time for premutation carriers compared with non-carriers.
METHODS
We determined AMH levels in blood samples from 240 women ascertained through fragile X families, of which 127 were premutation carriers and 113 were non-carriers. Linear mixed models were used to assess the effect of age and premutation status on AMH levels and to determine a modeled AMH value. The stability over time of the deviation of observed AMH levels from modeled levels, referred to as standardized AMH values, was assessed through correlation coefficients of 41 longitudinal samples.
RESULTS
At all ages, premutation carriers exhibited lower AMH levels. For all women, AMH was found to decrease by 10% per year. The added effect of having a premutation decreased AMH levels by 54%. The deviation of an individual's AMH level from the modeled value showed a reasonable intra-individual correlation. The Pearson correlation coefficient of two samples taken at different ages was 0.36 (P = 0.05) for non-carriers and 0.69 (P = 0.01) for carriers.
CONCLUSIONS
We developed a unique standardized AMH value, taking FMR1 premutation status and the subject's age into account, which appears to be stable over time and may serve as a predictor for FXPOI after further longitudinal assessment.
Keywords: anti-Müllerian hormone (AMH), premutation carriers, FMR1 gene, fragile X-associated primary ovarian insufficiency (FXPOI), ovarian reserve
Introduction
Age-related female fecundity depends on the reserve of the ovarian follicle pool (ovarian reserve) and the quality of the germ cells within this pool. With increasing age, female fecundity declines and fertility deteriorates until menopause occurs, around the age of 51 years (van Noord-Zaadstra et al., 1991; te Velde and Pearson, 2002). Some women already have a diminished ovarian reserve at a relatively young age, which is referred to as ovarian insufficiency. The spectrum of ovarian insufficiency ranges from diminished ovarian reserve to primary ovarian insufficiency (POI), previously referred to as premature ovarian failure, which is defined as amenorrhea for at least 4 months and post-menopausal FSH levels before the age of 40 years.
In past years, various markers, such as FSH serum levels or antral follicle count, have been used to measure ovarian insufficiency, but most of these markers only indicate advanced ovarian senescence. More recently, it was reported that the anti-Müllerian hormone (AMH) level may act as a useful marker for the ovarian follicle pool (van Rooij et al., 2004). Thus, AMH levels may serve as a marker for the ovarian follicle reserve during its natural decline (i.e. ovarian senescence) and, hence, for the risk of a low ovarian reserve at a young age (i.e. early ovarian senescence) and early exhaustion of the follicle pool (POI), which thereby can be identified earlier.
It has been well-documented that female carriers of a premutation in the fragile X mental retardation (FMR1) gene have an increased risk of early ovarian failure (Murray et al., 1998, 2000; Allingham-Hawkins et al., 1999; Sullivan et al., 2005), referred to as fragile X-associated primary ovarian insufficiency (FXPOI) (Welt, 2008). The premutation represents an expansion of a trinucleotide (CGG) repeat in the 5′ untranslated region of the FMR1 gene (Verkerk et al., 1991). The premutation (55–200 CGG repeats) can further expand to a full mutation with at least 200 CGG repeats, causing fragile X syndrome (MIM 309550), the most common heritable cause of intellectual and developmental disabilities. A normal allele with < 45 CGG repeats is stably inherited, but an intermediate allele (45–54 CGG repeats) may show instability upon transmission (Kronquist et al., 2008). Within the premutation range, a high risk for FXPOI has been suggested for ∼80–100 repeats (Sullivan et al., 2005; Ennis et al., 2006; Allen et al., 2007; Tejada et al., 2008). Women with a normal repeat length or a full mutation are not at risk for POI (Allingham-Hawkins et al., 1999; Murray et al., 1999). Recent studies suggest that repeat sizes between 35 and 55 may cause early ovarian senescence (Gleicher et al., 2009a,b; Gleicher and Barad, 2010); however, Bennett et al. (2010) failed to observe a significantly increased frequency of intermediate size repeat alleles among a large group of women with POI.
Recently, Rohr et al. (2008) measured AMH serum levels in premutation carriers in a cross-sectional study and found indications of reduced ovarian reserve even at early ages (18–30). AMH levels may serve as promising predictors of menopausal age, as is illustrated by a number of studies (Sowers et al., 2008; van Disseldorp et al., 2008; Tehrani et al., 2009). A recent study reported the development of a model to predict menopause based on AMH levels with consistent agreement between predicted and actual ages at menopause among naturally fertile women (Tehrani et al., 2010). As a corollary of the work reported by Rohr et al. (2008) and the high predictive value of AMH levels for menopause in the general population, we hypothesized that the decline in AMH is stable in carriers and non-carriers. If evidence supports this hypothesis, AMH may be used in the future to assess the ovarian reserve prior to early ovarian senescence. To test this hypothesis, we investigated the intra-individual longitudinal variation of AMH levels among premutation carriers and non-carrier women of fragile X families, adjusting for the premutation status and age.
Materials and Methods
Subjects
The study sample comprised participants from two centers, the Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands and the Emory University, Atlanta, GA, USA. After being informed about the objective and design of the present study, all participants provided informed consent. The study was approved by the institutional review boards (number NL23358.091.08; USA 100–2000)
The Nijmegen study
All fragile X families that were diagnosed at the Department of Human Genetics, Nijmegen between 1984 and 1998 were ascertained for this study. All female relatives of the proband aged 18–55 years were approached to participate in the present study, to complete a general health and reproductive history questionnaire and to supply a serum sample, as described in detail by Hundscheid et al. (2001). Briefly, participants with a natural menstrual cycle provided blood samples on the third day of their cycle (early follicular phase). For women using hormones, in all cases oral contraceptives (OCs), blood samples were obtained on the last day of the (7-day) pill-free interval. Blood samples taken on Day 3 of the menstrual cycle or on the last day of a hormone-free interval have been reported to show FSH levels comparable to those in the early follicular phase (Fauser and van Heusden, 1997; van Heusden and Fauser, 2002).
The Atlanta study
The Atlanta cohort from the Department of Human Genetics included women who were recruited through the Emory Study of Adult Learning and Reproduction. Ascertainment protocols have been described in detail by Sullivan et al. (2005) and Rohr et al. (2008). Briefly, to ascertain a large sample of varying repeat size (both intermediate and premutation alleles) both families with fragile X syndrome and women in the general population were surveyed. For both ascertainment strategies, females aged 18–75 were asked to complete a reproductive history questionnaire and to provide a buccal or blood sample for FMR1 CGG repeat size determination. In addition, for women who were still cycling or on hormone medication, blood samples were obtained on the third day of their cycle or of their pill-free interval, for the majority of samples. The most recent samples n= 60) were collected on any day of the menstrual cycle. For some samples, information on hormone use was unavailable and therefore the model could not be adjusted for hormone use.
Inclusion and exclusion criteria
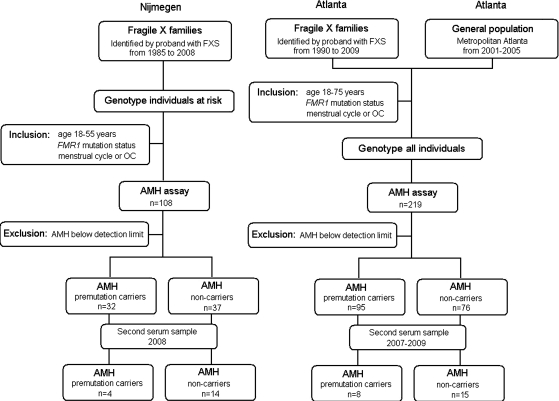
The following inclusion criteria were applied for participation in this study: (i) aged 18–55 years; (ii) FMR1 mutation status determined and (iii) menstruating at least once every 3 months or using hormones or OCs. After inclusion and AMH measurement, a fourth criterion was applied; namely undetectable AMH levels were excluded from analyses because they cannot be corrected for age and mutation status. Moreover, for this particular application to assess the stability of the standardized AMH, undetectable AMH levels cannot be used because they are unable to further decline over time. Women with iatrogenic menopause (after bilateral ovariectomy, radiation therapy or chemotherapy), ovarian surgery or polycystic ovary syndrome as defined by Rotterdam 2003 criteria (Rotterdam ESHRE/ASRM Sponsored PCOS Consensus Workshop Group, 2004) at any point in time were excluded from the study. The detailed study flowchart is shown in Fig. 1.
Figure 1.
Flowchart of the study sample by location.
Delineation of serum samples collected for both cohorts
For the Nijmegen cohort, stored serum samples collected in 1998 and 1999 (Hundscheid et al., 2001) were used. Sufficient amounts of frozen serum were available for AMH assays of 108 women from 50 unrelated fragile X families.
For the Atlanta cohort, stored serum samples were collected between 2002 and 2009 for FSH and AMH studies. Sufficient amounts of frozen serum were available for AMH assay of 219 women from 157 families. For a subset (115) of these women, AMH levels were determined and reported previously (Rohr et al., 2008). In total, 327 women from 180 families met the inclusion criteria and participated in this study, 108 samples from the Nijmegen cohort and 219 from the Atlanta cohort.
From all AMH values determined, 87 had an undetectable AMH level and were excluded from our study. Analyses were done on the 240 women with measurable AMH levels, of which 127 were premutation carriers and 113 non-carriers. For 41 of these 240 women, 12 premutation carriers and 29 non-carriers, a second serum sample, taken under the same conditions, resulted in AMH levels above detection limit. The time interval between the two samples varied from 1.5 year to 10 years.
Hormone assays
Serum derived from blood samples was stored at −35°C until use for the Nijmegen cohort. AMH levels were measured using an Enzyme-Linked Immunosorbent Assay (ELISA, Diagnostic System Laboratories, Inc./Beckman-Coulter). The detection limit of this assay was 0.10 ng/ml. Intra- and inter-assay coefficients of variation were < 5 and < 8%, respectively (La Marca et al., 2009).
For the Atlanta subset, serum samples were stored at −70°F until use. AMH levels were measured with the same Beckman-Coulter ELISA kit, with a detection limit of 0.05 ng/ml and similar intra- and inter-assay coefficients of variation.
FMR1 CGG repeat size measurement
From participants included in the Dutch study, DNA was extracted from peripheral blood cells. PCR amplification of the FMR1 alleles was performed using fluorescein (FAM)-labeled reverse primers as described by Fu et al. (1991). PCR conditions are available upon request. Fragment lengths were measured using an ABI prism 3730 DNA Analyser (Applied Biosystems) and quantified using ABI prism Genemapper Analysis Software v4.0 (Applied Biosystems).
From women participating in the Atlanta study, DNA was extracted from buccal samples or peripheral blood cells using Qiagen QiAmp DNA Blood Mini Kit. FMR1 CGG repeat sizes were determined by a fluorescent-sequencer method as described elsewhere (Meadows et al., 1996), using an ABI Prism 377 DNA Sequencer or the ABI 3100 Genetic Analyzer. For females showing only one FMR1 allele upon sequencing, a second PCR-based, hybridization technique was used to detect a possible larger fragment. The protocol used for this is a modified version of that developed by Brown et al. (1993). If no high repeat allele was detected using this follow-up strategy, we concluded that the woman in question was homozygous for the smaller allele.
Statistics
AMH serum levels exhibit a positively skewed distribution due to the age-related decline that falls below the detection limit. Natural logarithmic (log) transformations of AMH serum levels were applied to transform these distributions to normality. A linear mixed model was used to assess the effect of age and premutation status on AMH levels and to calculate a modeled AMH value based on age (continuous variable, years) and premutation status (dichotomous variable: non-carrier = 0, premutation carrier = 1). This model included a random family intercept to account for possible within family correlation. If AMH was measured at different occasions for a subject, only the first observation was used to develop this model.
For every individual, the modeled logarithmic transformed AMH value was defined by the regression equation:
where 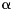 and
and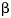 represent the estimated effects of age and premutation, respectively. The deviation of an individual's observed log(AMH) level from the modeled y1 value is defined as
represent the estimated effects of age and premutation, respectively. The deviation of an individual's observed log(AMH) level from the modeled y1 value is defined as
Standardized AMH = [observed log(AMH)–modeled log(AMH)]/residual standard deviation,
where the residual standard deviation results from fitting the linear model to the first observed AMH values. For subjects with two AMH values observed at different ages, we determined the second standardized AMH value using the coefficients from the regression equation fitted on the first observations as described earlier, but now using the observed AMH level taken at the second time point. The Pearson correlation coefficient between the first and second standardized AMH values and scatterplots were used to examine the stability of the standardized AMH values over time within subjects.
Apart from the assessment of stability of standardized AMH, the difference in AMH levels between premutation carriers and non-carriers was determined. In order to compare AMH levels from premutation carriers and non-carriers, we modeled the best fitting curves for both groups on the full set of AMH levels, including first and second AMH samples. The best fitting curves were constructed by a multilevel mixed linear model, accounting for the clustering of observations within a subject and clustering of subjects within a family. All models were based on the logarithmic transformed AMH values using age and premutation status as independent variables.
Analyses using linear mixed models were carried out with Statistical Analysis Software (SAS), version 8.2. Statistical significance was considered to be present at P< 0.05.
Results
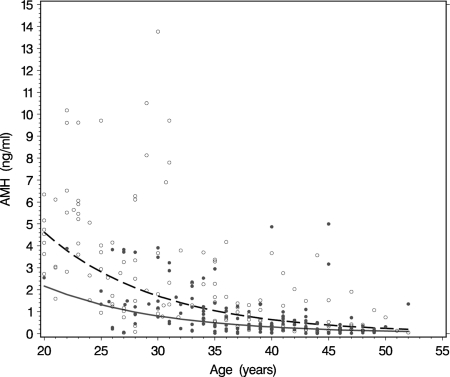
Analyses were performed on the total data set of 240 women with detectable AMH serum levels. The median age at which the blood samples were taken was 37 (range 18–55) years for premutation carriers and 31 (range 20–51) years for non-carriers. The median ages and repeat sizes per location are provided in Table I. The distribution of AMH serum levels by age for both premutation carriers and non-carriers confirmed the previously described (Rohr et al., 2008) lower ovarian reserve for premutation carriers at all ages (Fig. 2).
Table I.
The median ages and repeat sizes by location.
| Number of patients | Median age (years) [p25–p75] | Median repeat lengtha [p25–p75] | |
|---|---|---|---|
| Carriers Nijmegen | 32 | 37 [34–41] | 89 [82–99] |
| Carriers Atlanta | 95 | 34 [30–41] | 90 [79–100] |
| Non-carriers Nijmegen | 37 | 36 [31–40] | 31 [29–39] |
| Non-carriers Atlanta | 76 | 28 [23–39] | 41 [30–44] |
aThe median repeat length of the largest allele.
Figure 2.
Scatterplot of AMH levels by age for premutation carriers (closed circles) and non-carriers (open circles). The best fitting curves (solid line for premutation carriers and dashed line for non-carriers) were constructed by a multilevel mixed linear model, accounting for the clustering of observations within subjects and clustering of subjects within families, on the logarithmic transformed AMH values. For this model we used as independent variables age and premutation status.
Development of modeled AMH values
In order to correct AMH serum levels for effects of the premutation and age on the ovarian reserve, a modeled AMH value was developed using linear mixed model analyses. By doing so, we found a statistically significant effect on our outcome measure, observed log(AMH), of −0.11 for each year a person aged (P < 0.0001) and −0.78 for premutation carrier status (P < 0.0001). Our model yielded the following equation to determine the modeled AMH value:
The random intercept allowing within family correlation was 0, and the residual standard deviation was 1.133. These modeled log(AMH) values were transformed back to the original scale for interpretation to describe whether the observed AMH values were above or below the modeled AMH values, implying a larger or lower ovarian reserve than expected for an individual's age and premutation status.
Deviation from modeled AMH: standardized AMH is stable over time
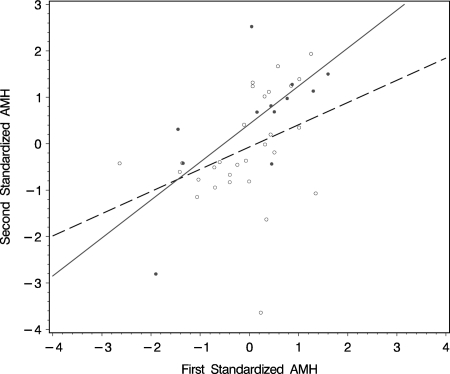
The interpretation of an individual AMH level was quantified by the deviation of the observed measured log(AMH) level from the modeled log(AMH) values divided by the residual standard deviation, which is termed 'standardized AMH value’. The standardized AMH values ranged from −2.9 to 2.7 for premutation carriers and from −3.6 to 1.9 for non-carriers. Women with an additional second sample were used to assess the stability of the decline in AMH levels over time. The correlation coefficients of the standardized AMH values derived from the two time points was reasonable, with a Pearson correlation coefficient of 0.36 with 95% confidence interval (CI): (−0.005 ; 0.64), (P = 0.05, n = 29) for non-carriers and 0.69 with 95% CI:(0.19 ; 0.91), (P = 0.01, n = 12) for premutation carriers (Fig. 3). This reasonable conformity is indicative of a small intra-individual variation in standardized AMH values and stability over time, both attributes being beneficial to a possible predictor for menopause in premutation carriers.
Figure 3.
Correlation of standardized AMH values in subset of premutation carriers (closed circle; R = 0.69, P = 0.01) and non-carriers (open circle; R = 0.36, P = 0.05) with two AMH measurements at different ages as shown on a linear regression curve (solid line for premutation carriers and dashed line for non-carriers).
Age-related decline in AMH
The age-related decline in AMH, and the difference in decline between premutation carriers and non-carriers were assessed using a multilevel mixed linear model (see Materials and Methods). On the logarithmic scale, this model indicates a premutation effect of −0.76 (P < 0.0001), which means that at each age the value of AMH in a premutation carrier is on average 46% [exp(−0.78)] of that in a non-carrier of the same age. For both carriers and non-carriers, the effect of aging is a decrease of 10% [exp(−0.11)] in AMH levels per year, starting at the age of 18 years. Figure 2 shows the results on the original AMH scale.
Discussion
This is the first study to assess age and premutation-corrected AMH serum levels in premutation carriers who are at risk for FXPOI. Using these data, we propose a standardized AMH that is stable over time in premutation carriers and non-carriers. Several steps were taken to develop this standardized variable. First, a large data set was derived by pooling samples from two clinical centers addressing FMR1 premutation carriers and their reproduction. The collaboration also resulted in a more representative sample with regard to age and repeat size ranges. Second, a model was developed to determine an individual's modeled AMH based on age and premutation status by linear mixed model analysis. Overall, we found that both age at AMH measurement and premutation carrier status played a large role in modeled AMH levels. Using the standardized AMH levels, we found that the correlation obtained at two time points was higher for premutation carriers compared with non-carriers. We think that this can be explained by three non-carrier women exhibiting a standardized AMH above their modeled value at first sample and far below their modeled AMH value at second sample (Fig. 3). Possible differences in hormonal status, like pregnancy or OC use may play a role and should be considered when AMH levels are measured.
To date, age-related decline in AMH serum levels has been studied for the general population and some subfertile populations in relation to IVF (La Marca A. et al., 2010). AMH serum levels as a function of age have shown wide variation, as depicted by Tremellen et al. (2005). In their study on prediction of menopause, Van Disseldorp et al. (2008) modeled mean decline of AMH with age and applied a menopausal threshold AMH level to correlate this predictive distribution with the distribution of observed age at menopause. There was a good level of conformity between the two distributions. Although their modeled AMH level was based on a cross-sectional survey, the results are highly suggestive of AMH being a good predictor for menopausal age.
Few studies have addressed AMH decline by a longitudinal setup. De Vet et al. (2002) showed a significant decrease over time of an individual's AMH level and a high correlation with age. Van Rooij et al. (2005) expanded AMH as a marker for ovarian reserve a step further and assessed the consistency of AMH over time in a comparable manner to this study. They investigated whether a woman's individual level above or below the mean of her age group at time point 1 remained above or below the mean of her age group at time point 2. Serum AMH levels showed the best consistency compared with other ovarian reserve markers. Our current study took another step by correcting for age and assessing whether the consistency seen in normo-ovulatory women is also seen in premutation carriers, adjusting for the fact that carriers are known to be at risk for a lower ovarian reserve.
Although there is evidence that AMH may serve as a potential FXPOI predictor, we have taken into account that the results are based on a relatively small longitudinal sample size, in spite of pooling samples from two clinical centers. There are several other directions that should be taken in future studies that we were not able to accommodate in this study. First, in our linear mixed model analyses, the dichotomized variable of premutation status was used to adjust for the risk of FXPOI. However, we know that there is a nonlinear association of risk for FXPOI and the size of the premutation, but we were unable to incorporate this effect due to our small sample size. We think that taking into account the nonlinear risk will provide better adjustments for the risk of low ovarian reserve for premutation carriers and, therefore, should be investigated further. Not only the size of premutation alleles, but also the intermediate repeat sizes could be involved in ovarian function and cause early ovarian senescence or premature ovarian aging as was recently shown (Gleicher et al., 2009a,b; Gleicher and Barad, 2010; Gleicher et al., 2010). However comparison of AMH levels in our women with an intermediate repeat sizes (35–45 or 46–55 repeats; n= 49) with AMH levels of repeat sizes < 35, does not show lower AMH levels among women with intermediate CGG repeats (18–30 years, P = 0.25; 31–40 years, P = 0.67; >40 years, P = 0.48, Kruskal–Wallis test).
Second, we did not correct for use of hormones or the phase of the menstrual cycle at the time the blood sample was taken. Instead, we assessed the age distribution of samples taken during OC use and found a similar normal distribution for premutation carriers and non-carriers, implying that an effect of OC use on the modeled AMH values is unlikely. Furthermore samples taken at any time in the menstrual cycle were also normally distribution by age for premutation carriers and non-carriers. At first AMH levels were considered independent of hormonal variations (Hehenkamp et al., 2006; La Marca et al., 2006; Somunkiran et al., 2007). More recently, however, several studies have questioned this independency. Van den Berg et al. (2010) showed a significant difference in both AMH and FSH levels in a natural cycle and the subsequent first and second hormone-free intervals. Also, circulating AMH levels have been shown to decline during the second and third trimester of pregnancy (Nelson et al., 2010).
The lower AMH levels among premutation carriers seen at all ages also emphasize the need for ways to enhance early identification of low ovarian reserve to facilitate counseling for FXPOI and concomitant family planning. McConkie-Rossell et al. (2005) have suggested that monitoring of ovarian function should be recommended when counseling premutation women, even though an exact delineation of the onset of FXPOI is difficult. Early identification of premutation carriers who will develop FXPOI is expected to lead to better chances of pregnancy, either spontaneous or by IVF. When pregnancy cannot be established due to lack of a partner or is postponed for other reasons, cryopreservation of oocytes by vitrification has recently been presented as a realistic option for fertility preservation for young premutation carriers.
In conclusion, we have developed an attractive method, using standardized AMH values, which may be suitable as a potential predictor for menopausal age in FMR1 premutation carriers at risk for FXPOI. Further assessment of our standardized AMH value by a large longitudinal sample and association with menopausal age will be needed to demonstrate its benefit as a predictor for FXPOI.
Authors' roles
M.A.S. acquired data, performed analysis and interpretation of data, and wrote the article including the version submitted.
T.B.F. analyzed and interpreted the data, revised the drafts and approved the version to be submitted. E.G.A. acquired data and contributed to study follow-up, critically revised the drafts and approved final version to be submitted. A.P.T.S. contributed to the concept and design of study, critically revised the drafts and approved final version to be submitted. H.G.Y. analyzed molecular genetic data, revised the content of the article and approved submission. A.H.M.G.K. contributed to the concept and design of the study, revised the content of the drafts and approved the version to be submitted. D.D.M.B. contributed to the concept of the study, revised the content and approved the version to be submitted. S.L.S. contributed to the concept and design of the study and interpretation of data, critically revised the drafts and approved final version to be submitted. C.M.G.T. contributed substantially to the design of the study and interpretation of data, critically revised the drafts for intellectual content and approved the version to be submitted.
Funding
This work was supported in part by National Institutes of Health grants NIH RO1 HD29909 and NIH PO1 HD35576 (EGA, SLS)
Acknowledgements
The authors thank all the women who participated in this study and Rob van der Steen for excellent technical assistance.
References
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, Holden JJ, Yang KT, Lee C, Hudson R, Gorwill H, Nolin SL, Glicksman A, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the International Collaborative POF in fragile X study-preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25:1335–1338. doi: 10.1093/humrep/deq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WT, Houck GE, Jr, Jeziorowska A, Levinson FN, Ding X, Dobkin C, Zhong N, Henderson J, Brooks SS, Jenkins EC. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993;270:1569–1575. [PubMed] [Google Scholar]
- de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Anti-Mullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC, van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev. 1997;18:71–106. doi: 10.1210/edrv.18.1.0290. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG, Jr, Warren ST. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Barad DH. The FMR1 gene as regulator of ovarian recruitment and ovarian reserve. Obstet Gynecol Surv. 2010;65:523–530. doi: 10.1097/OGX.0b013e3181f8bdda. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009a;19:385–390. doi: 10.1016/s1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand. 2009b;88:1024–1030. doi: 10.1080/00016340903171058. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. Reprod Biomed Online. 2010;20:768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- Hundscheid RD, Braat DD, Kiemeney LA, Smits AP, Thomas CM. Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod. 2001;16:457–462. doi: 10.1093/humrep/16.3.457. [DOI] [PubMed] [Google Scholar]
- Kronquist KE, Sherman SL, Spector EB. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med. 2008;10:845–847. doi: 10.1097/GIM.0b013e31818b0c8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24:2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- La Marca A., Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Finucane B, Cronister A, Abrams L, Bennett RL, Pettersen BJ. Genetic counseling for fragile X syndrome: updated recommendations of the national society of genetic counselors. J Genet Couns. 2005;14:249–270. doi: 10.1007/s10897-005-4802-x. [DOI] [PubMed] [Google Scholar]
- Meadows KL, Pettay D, Newman J, Hersey J, Ashley AE, Sherman SL. Survey of the fragile X syndrome and the fragile X E syndrome in a special education needs population. Am J Med Genet. 1996;64:428–433. doi: 10.1002/(SICI)1096-8628(19960809)64:2<428::AID-AJMG39>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Murray A, Webb J, Grimley S, Conway G, Jacobs P. Studies of FRAXA and FRAXE in women with premature ovarian failure. J Med Genet. 1998;35:637–640. doi: 10.1136/jmg.35.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE, Conway GS. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod. 1999;14:1217–1218. doi: 10.1093/humrep/14.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Ennis S, MacSwiney F, Webb J, Morton NE. Reproductive and menstrual history of females with fragile X expansions. Eur J Hum Genet. 2000;8:247–252. doi: 10.1038/sj.ejhg.5200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Stewart F, Fleming R, Freeman DJ. Longitudinal assessment of anti-Mullerian hormone during pregnancy-relationship with maternal adiposity, insulin, and adiponectin. Fertil Steril. 2010;93:1356–1358. doi: 10.1016/j.fertnstert.2009.07.1676. [DOI] [PubMed] [Google Scholar]
- Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Somunkiran A, Yavuz T, Yucel O, Ozdemir I. Anti-Mullerian hormone levels during hormonal contraception in women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2007;134:196–201. doi: 10.1016/j.ejogrb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr Anti-Mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of anti-Mullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/gme.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- Tehrani FR, Shakeri N, Azizi F. Whether age at menopause is predictable using serum anti-Mullerian hormone concentration? Human Reproduction. 2010;25:i2. [Google Scholar]
- Tejada MI, Garcia-Alegria E, Bilbao A, Martinez-Bouzas C, Beristain E, Poch M, Ramos-Arroyo MA, Lopez B, Fernandez I, Ribate MP, et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause. 2008;15:945–949. doi: 10.1097/gme.0b013e3181647762. [DOI] [PubMed] [Google Scholar]
- Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-Mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol. 2005;45:20–24. doi: 10.1111/j.1479-828X.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- van den Berg MH, van Dulmen-den BE, Overbeek A, Twisk JW, Schats R, van Leeuwen FE, Kaspers GJ, Lambalk CB. Comparison of ovarian function markers in users of hormonal contraceptives during the hormone-free interval and subsequent natural early follicular phases. Hum Reprod. 2010;25:1520–1527. doi: 10.1093/humrep/deq071. [DOI] [PubMed] [Google Scholar]
- van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. Relationship of serum anti-Mullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–2134. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- van Heusden AM, Fauser BC. Residual ovarian activity during oral steroid contraception. Hum Reprod Update. 2002;8:345–358. doi: 10.1093/humupd/8.4.345. [DOI] [PubMed] [Google Scholar]
- van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302:1361–1365. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij I, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, Themmen AP, te Velde ER. Anti-Mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- van Rooij I, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. Serum anti-Mullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83:979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]