Abstract
Aims
Fructose induces metabolic syndrome in rats but studies have been criticized for using high concentrations of fructose that are not physiologic, for using only pure fructose, and for not controlling for energy intake. We tested the hypothesis that a 40% sucrose diet (containing 20% fructose) might induce features of metabolic syndrome in male breeder rats independent of excess energy intake.
Methods
Male Sprague-Dawley breeder rats were pair fed 40% sucrose or isocaloric starch diet for 4 months and evaluated for metabolic syndrome and diabetes. In vitro studies were performed in rat insulinoma cells (RIN-m5F) exposed to uric acid and markers of inflammation were assessed.
Results
Rats fed a 40% sucrose diet developed accelerated features of metabolic syndrome with upregulation of fructose-dependent transporter Glut 5 and fructokinase. Fatty liver and low grade pancreatic inflammation also occurred. Uric acid was found to stimulate inflammatory mediators and oxidative stress in islet cells in vitro.
Conclusions
Sucrose, at concentrations ingested by a subset of Americans, can accelerate metabolic syndrome, fatty liver and type 2 diabetes in male breeder rats, and the effects are independent of excess energy intake.
Keywords: Sucrose, Fructose, Insulin Resistance, Uric acid, Nonalcoholic Fatty Liver Disease
Introduction
Diets enriched in fructose are known to induce features of metabolic syndrome in rats [1–2]. However, these studies are frequently criticized for using excessive concentrations of fructose that are not physiological (e.g., 60%) or for administering fructose alone (when most exposure to humans is as sucrose or high fructose corn syrup) [3–4]. Furthermore, there remains debate whether the effects of fructose to induce metabolic syndrome are simply a consequence of excessive energy intake [5]. Finally, while there is some data that added sugars may increase the risk for type 2 diabetes both experimentally [6] and in humans [7–8], there is only minimal evidence that they have specific effects to induce islet cell dysfunction [9–11].
We therefore tested the hypothesis that a sucrose-based diet containing only 20% fructose might induce features of fatty liver and metabolic syndrome. To address whether the effects observed were specific to fructose, we administered an identical diet to control rats in which the sucrose was replaced with starch. Furthermore, all rats were pair fed so that each animal ate the exact same number of calories. To further assure that intake was not excessive, all rats were fed approximately 90% of normal intake. The slight restriction in caloric intake is to simulate a dieting individual who still ingests a diet high in added sugars. Studies were performed in male Sprague Dawley breeder rats, which are known to spontaneously develop features of metabolic syndrome as they age [12–13]. Using this model system, we report that sucrose accelerated the development of metabolic syndrome and type 2 diabetes compared to starch fed rats, in association with the development of mild inflammation in the pancreas.
Methods
Animals and Diets
Eight month-old male Sprague-Dawley breeder rats (Charles Rivers, Wilmington, MA) were housed in the animal facility at the University of Colorado. Rats were kept under temperature- and humidity-controlled specific pathogen-free conditions and maintained on a 12 hour light-dark cycle. The experimental protocols were approved by the University of Colorado Animal Care and Use Committee.
Rats were randomly divided into two groups, consisting of sucrose-fed (n=10) or starch-fed (n=10) rats. The sucrose group received a 40% sucrose diet (protein 18.3 % by weight and 20.2% Kcal; carbohydrate 60.5% by weight (consisting of 40% sucrose and 20.5% starch) and 66.9% Kcal; fat 5.2 % by weight and 12.9% Kcal; TD.09019 Harlan). The control group received an isocaloric (based on weight) diet in which the sucrose was replaced by starch (Selected Nutrient; protein 18.3 % by weight and 20.2% Kcal; carbohydrate 60.5% by weight and 66.9% Kcal; fat 5.2 % by weight and 12.9% Kcal, TD.06391 Harlan). The diets were identical in all nutrients other than replacing the starch for sucrose in the control diet (Table 1).
Table 1.
Composition of the Diets
| Sucrose Diet (g/kg) | Control Diet (g/kg) | |
|---|---|---|
| Casein | 207.0 | 207 |
| DL-Methionine | 3.0 | 3.0 |
| Corn Starch (glucose polymer) | 12.0 | 456 |
| Maltodextrin | 200.0 | 200.0 |
| Sucrose | 400.0 | 0 |
| Lard | 50.0 | 50.0 |
| Cellulose | 60.21 | 16.36 |
| Mineral Mix, Roger-Harper(170760) | 50.0 | 50.0 |
| Zinc Carbonate | 0.04 | 0.04 |
| Vitamin Mix, Teklad (40060) | 10.0 | 10.0 |
| Potassium Chloride | 7.6 | 7.6 |
| Pink food color | 0.15 | |
All rats were placed in individual cages and pair fed the same amount of total calories each evening for the 16 weeks. Food intake was measured daily, and averaged approximately 21 g/d. This represented about 10% less than normal intake in these rats. Rats generally ate about 9 g in the first two hours and then the rest of their food over the following 18 to 20 hours. All food was ingested by 24 hours.
Blood Sampling
Fasting (8 hour) blood samples were obtained from the tail vein from unanesthetized rats at baseline, 1 month and 4 months. Serum was analyzed for glucose using the Glucose Assay Kit (Cayman Chemical Company, Ann Arbor, MI). Triglycerides and uric acid using the Vetace autoanalyzer (Alfa Wassermann, West Caldwell, NJ) Serum insulin was measured by ELISA (Crystal Chemical, Chicago, IL). Animal body weights were measured weekly.
Blood Pressure
Blood pressure (BP) was measured by intraaortic telemetry in conscious, unrestrained rats using the DSI telemetry system. Ten days prior to initiating the study a BP sensor (model TA11PA-C40, DSI Inc, St Paul, MN) was inserted into the aorta below the level of the renal arteries, and the radiofrequency transmitter fixed to the peritoneum. For BP measurements a plastic cage containing the rat was placed on top of a Lab Pro Data Acquisition System receiver, and systolic BP was continuously recorded at 5-minute intervals with each BP reading representing the average of ~60 readings during a 10 second interval.
Liver Oil Red O Stain
Liver tissue collected under isofluorane anesthesia was embedded in Optimal Cutting Temperature gel (OCT, Sakura Finetek, Torrance, CA) and frozen in liquid nitrogen. Air-dried cryostat tissue sections (8μm) were dipped in formalin, washed with running tap water, rinsed with 60% isopropanol, and stained with Oil Red O counterstained with haematoxylin. Macrovesicular fat deposition was defined as the presence of lipid vacuoles that are larger than the nucleus and usually displaces it to the periphery of the cell.
Pancreatic Histology and Immunostaining
The pancreas was dissected under isofluorane anesthesia, fixed in Methyl Carnoy’s solution, embedded in paraffin, and sections (3μm) and stained with periodic acid Schiff (PAS). Immunostaining was performed using our standard procedure [14] with the following antibodies: mouse anti-rat Insulin (Cell Signaling Technology-Danvers, MA), ED-1 (mouse antibody to rat monocytes and macrophages, Serotec, Raleigh, NC), and rat URAT-1 (Murine anti-SLC22A12/Urat-1, ABNOVA, Walnut, CA). A horse radish peroxidase conjugated secondary antibody was used followed by detection with diaminobenzidine-H2O2 (DAB) (Vector, Burlingame, CA). For quantification, at least 5 islets were examined per biopsy. Images were captured using the ScanScope Scanner Console and Aperio Image Scope software; (APERIO Technologies, Inc., Vista, California) and by color saturation the percent positive immunostaining area quantified. Infiltration of macrophages in pancreas interstitium was assessed by quantifying the percent positive area in 5 grids not containing islets for each biopsy. Mean scores for the rats from each group were compared. All studies were analyzed blinded.
Western blotting of liver and pancreas
Protein lysates were prepared from liver and pancreatic tissues as previously described [15]. Sample protein content was determined by the BCA protein assay (Pierce, Rockford, IL). 50 μg of total protein was loaded per lane for SDS-PAGE (10% w/v) analysis and transferred to PVDF membranes. Membranes were incubated with the following primary antibodies: fatty acid synthase (FAS, Cell signaling, Danvers, MA), fructokinase isoform C (KHK-C) (Sigma, St Louis, MO). AMP Deaminase 2 (AMPD2, Abcam, Cambridge, MA), Enolyl CoA hydratase (ECoAH1, ProteinTech Group, Chicago, IL) URAT-1 (Murine anti-SLC22A12/Urat-1, ABNOVA, Walnut, CA) and β-actin (Cell signaling). Blots were then incubated with a horseradish peroxidase secondary antibody and Immunstar HRP chemiluminescence kit (BioRad) as described by the manufacturer. Chemiluminescence was recorded with an Image Station 440CF and results analyzed with the 1D Image Software (Kodak Digital Science, Rochester, NY).
RNA extraction, analysis and message quantification
Total RNA was isolated from liver, pancreas and RIN-m5F cells using the RNeasy kit (Qiagen, Valencia, CA). and integrity assessed as the 28S to 18S rRNA ratio by capillary electrophoresis. RNA was converted to cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCR primers specific to rat KHK-C (For GGTGTGGATGTGTCTCAAGTG, Rev TGGCAGGTTCGTGTC), FAS (For GCGAGTCTATGCCACTATTC, Rev AGCTGATACAGAGAACGGATG), ATP citrate lyase (For CAGTGAACAACAGACCTATGAC, Rev CAATGCTGCCCTCCAATGATG), chemokine (C-X-C motif) ligand 1 (KC or CXCL-1) (For GATGGCGTCTGTCTGGTG, Rev AGGACCCTCAATAGAAATCGTAAA), Monocyte chemoattractant protein-1 (MCP1) (For GCATCAACCCTAAGGACTTCA, Rev GCATCACATTCCAAATCACACT), interleukin-6 (IL6) (For GGACCAAGACCATCCAACTC, Rev CAACATTCATATTGCCAGTTCT), insulin (For ACAGCACCTTTGTGGTCC, Rev GGACTCAGTTGCAGTAGT) and β-actin (For TATCGGCAATGAGCGGTTC, Rev AGCACTGTGTTGGCATAGAG), were designed using Beacon Designer 5.0 software (Premier Biosoft International, Palo Alto, CA). Quantitative PCR was performed using the primers at 70 nM and the SYBR Green JumpStart® Taq Readymix® qPCR kit (Sigma) on a BioRad I-Cycler. Quantitative PCR runs were analyzed by agarose gel electrophoresis and melt curve to verify the correct amplicon was produced. β-actin RNA was used as internal control and the amount of RNA was calculated by the comparative CT method as recommended by the manufacturer.
Cell culture studies
The established rat insulinoma cell line RIN-m5F (American Type Culture Collection, location) were grown in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin. To test the effects of uric acid, the media in culture dishes were supplemented with uric acid (0–6 mg/dl) for different time points. Water soluble probenecid (Molecular Probes, location) was employed at 2 mM concentration. Experiments were initiated once cells reached confluence in 24-well flat bottom tissue culture plates (35–3047; Falcon BD Labware, Franklin Lakes, NJ), with each experimental time point after exposure to uric acid performed in triplicate.
Determination of intracellular oxidative stress in RIN-m5F cells
Reactive oxygen species in live RIN-m5F cells plated in 96-well plates was determined employing the Image-iT™ LIVE Green Reactive Oxygen Species (ROS) Detection Kit I36007, Molecular Probes) as per manufacturer’s instructions. Briefly, cells were grown to 90 % confluency in presence or absence (control) of uric acid (3 or 6 mg/dl) alone or in combination with 2 mM probenecid for 24 hours. After treatment, cells were washed with warm PBS and incubated with 25 μM of the cell-permeant fluorescent probe carboxy H2-dichlorodihydrofluorescein (DCF) for 30 minutes at 37°C protected from light. After incubation cells were washed with warm buffer and total fluorescence was determined employing a multi mode microplate reader (Biotek synergy 2, Winooski, VT) with the following settings: excitation (458 ± 20 nm), emission (528 ± 20 nm). DCF fluorescence signal was expressed as total fluorescence intensity normalized to miligram of soluble protein.
Statistical Analysis
Data are presented as least square mean ± standard error (S.E) unless otherwise noted. The starch and sugar treated groups were compared using mixed model repeated measures analysis with pre-planned contrasts in order to take into account correlated measurements for each rat. Within group comparisons were also analyzed using mixed models in paired analyses. At 4 weeks data were obtained on 5 rats in each group, but estimates were obtained using PROC MIXED in SAS (9.2). Significance was defined as a p value < 0.05 for pre-planned contrasts.
Results
Rats were pair-fed isocaloric diets containing sucrose or starch for 4 months. . Since all rats were pair fed, all rats received the identical energy intake.
Effect of Sucrose diet on Fructose Transport and Metabolism
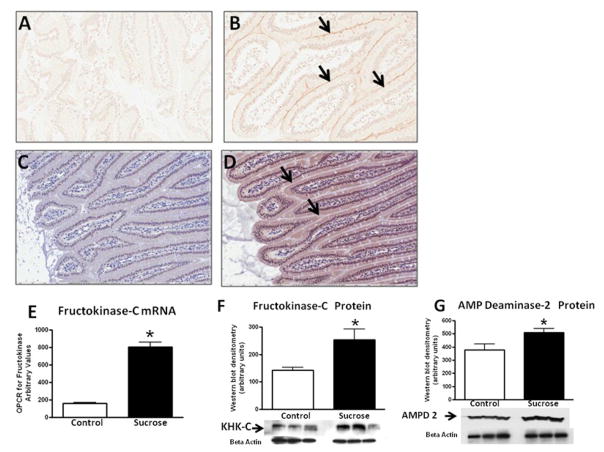
Diets high in fructose (50–60%) upregulate Glut5 (fructose transporter in the gut) and fructokinase in the intestine and liver [16–19]. As shown in Figure 1, rats fed 40% sucrose (which contains 20% fructose) showed increased Glut5 (jejunum) and fructokinase C expression in their jejunum and liver. AMP deaminase is stimulated during fructose metabolism in vitro, which eventually leads to the generation of uric acid [20]. The expression of AMP deaminase isoform 2 (the major hepatic isoform) was significantly increased in rats on the sucrose diet (p<0.05, Fig 1). Thus, a 40% sucrose diet upregulates transporters and enzymes involved in fructose metabolism.
Figure 1. Expression of Glut 5 and Fructokinase.
Jejunal tissues obtained from starch and sucrose-fed rats were examined for the fructose transporter, Glut 5, and for fructokinase-C. In contrast to control rats (A), immunostaining showed the expression of Glut 5 along the apical border (B, light brown line highlighted by arrows). Compared to control rats (C), fructokinase was also increased in the cytoplasm of the sucrose-fed rats (D, brown colored cytoplasm, see arrows). Sucrose fed rats showed an increased hepatic expression of fructokinase mRNA (E), fructokinase protein (F) and AMP Deaminase 2 protein (G) compared to starch-fed rats. A and B, 40 x; C and D, 20x *p<0.05.
Effect of Sucrose Diet on Metabolic Syndrome
At baseline mean blood sugar, fasting insulin levels, and serum triglycerides were in the normal range in both groups (Table 2). At 4 weeks control rats developed fasting hyperinsulinemia, although serum glucose and triglycerides remained in the normal range (Table 2). However, sucrose-fed rats developed significantly higher levels of triglycerides, serum insulin and uric acid values, consistent with an insulin resistant state. At 16 weeks control rats showed borderline elevated blood glucose levels with hyperinsulinemia and hypertriglyceridemia. Sucrose fed rats had significantly higher blood glucose levels; however, the striking finding was that serum insulin levels had fallen to levels below that observed in the control rats (Table 2).
TABLE 2.
Metabolic Parameters at Various Times Following Diet
| Baseline | Control | Sucrose-Fed | p values |
|---|---|---|---|
| Body weight (g) | 606 ± 11 | 590 ± 11 | NS |
| Fasting blood glucose (mg/dL) | 94 ±10 | 95±11 | NS |
| Serum Uric Acid (mg/dL) | 2.8 ± 0.2 | 3.0 ± 0.2 | NS |
| Serum triglyceride (mg/dL) | 111 ± 19 | 101 ± 19 | NS |
| Serum insulin (pg/ml) | 248 ± 93 | 234± 94 | NS |
| 4 Weeks | |||
| Body weight (g) | 606 ± 11 | 596 ± 11 | NS |
| Fasting blood glucose (mg/dL) | 82 ± 14 | 108 ± 14 | NS |
| Serum Uric Acid (mg/dL) | 2.6 ± 0.2 | 3.5 ± 0.2* | 0.007 |
| Serum triglyceride (mg/dL) | 121± 26 | 287 ± 26* | 0.0002 |
| Serum insulin (pg/ml) | 754 ± 119* | 1151 ± 119* | 0.03 |
| 16 Weeks | |||
| Body weight (% change) | −0.85 ± 1.3 | 2.69 ± 1.7 | NS |
| Body weight (g) | 601 ± 11 | 604 ± 11 | NS |
| Fasting blood glucose (mg/dL) | 124 ± 11 | 181 ± 14* | 0.001 |
| Serum Uric Acid (mg/dL) | 2.2 ± 0.2* | 2.3 ± 0.2* | NS |
| Serum triglyceride (mg/dL) | 165 ± 19 | 165 ± 21 | NS |
| Serum insulin (pg/ml) | 1198 ± 100* | 801 ± 100* | 0.01 |
p<0.05 within group comparisons to baseline. Data are presented as least square mean ± SE.
Mean body weights were not different between control and sucrose groups at any time during the study (Table 2).
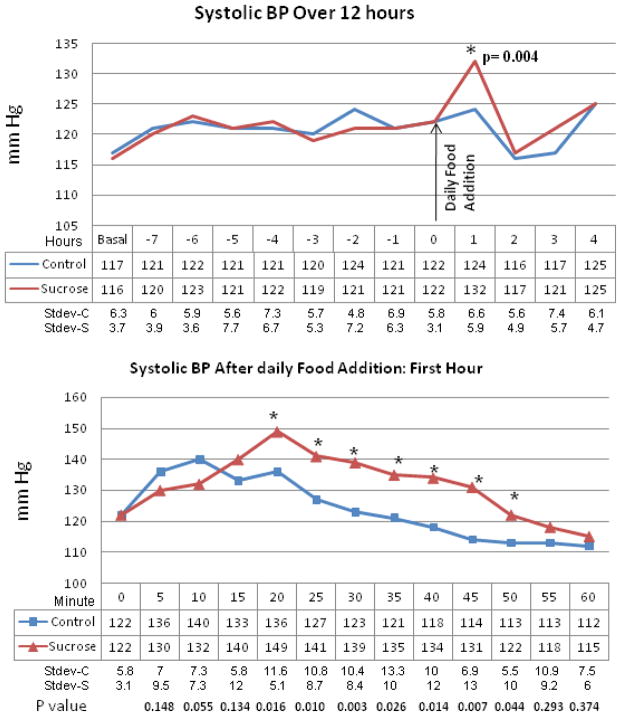
Blood pressure was measured in conscious unrestrained rats using an aortic telemetry system. Mean 24 hour blood pressure, measured at monthly intervals, were not different between groups. However, when hourly blood pressure measurements were obtained at each of these time points, a consistent increase in systolic blood pressure was observed in the sucrose fed rats during the first hour after food was provided (Fig 2).
Figure 2. Systolic Blood Pressure in Control versus Sucrose Fed Rats.
Upper panel: Systolic blood pressures as measured by telemetry over a 12 hour period. Mean blood pressures remain no different between control (starch fed, blue line, n=8) and sucrose-fed (red line, n=9) rats except for the first hour after introduction of food. Lower panel: Serial blood pressures during the first hour after exposure to food shows significant increases in blood pressure in the sucrose fed rats. *p=0.004 Shown are measurements obtained at 2 weeks after initiating the diet.
Sucrose Diet Induces Fatty Liver
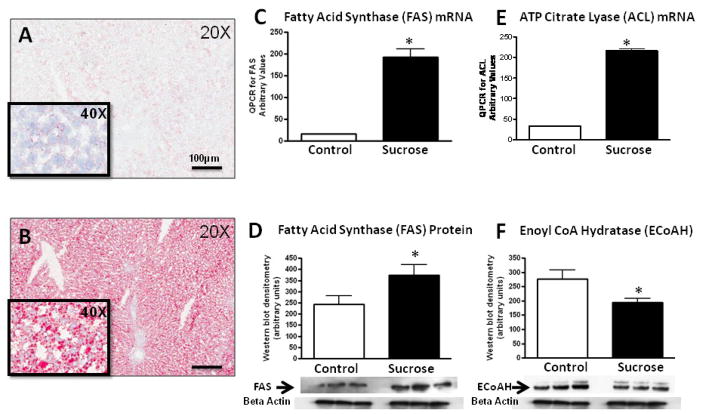
Diets high in fructose (60%) induce fatty liver in male Sprague Dawley rats, but the effects of lower concentrations of fructose on fatty liver is unknown. As shown in Figure 3, sucrose-fed rats developed macro- and microvesicular steatosis as noted by Oil Red O stain. Previous studies have suggested that sucrose and fructose induce both an increase in fat synthesis and a decrease in fat oxidation [21–22]. Consistent with this observation, western blotting livers of sucrose-fed rats showed increase in fatty acid synthase (FAS) and ATP citrate lyase (ACL) (involved in fat synthesis) and decreases in enoyl CoA hydratase (ECoAH1) (involved in β fatty acid oxidation (Fig 3).
Figure 3. Effects of Diet on Fatty Liver.
Rat liver tissue obtained at 4 months shows negative staining for fat by Oil Red O stain in starch-fed, control rats (A) whereas diffuse micro and macrovesicular fat deposits are present in sucrose-fed rats (B). QPCR analysis of whole liver tissues shows an increase in Fatty acid synthase (C) and ATP Citrate Lysate mRNA (D) in Sucrose-fed rats. Western blots confirmed an increase in Fatty Acid Synthase protein (E) and a decrease in Enoyl CoA hydratase-1 protein in sucrose-fed rats (F). Key, A and B, Oil Red Stain, 20X magnification. *, p<0.05
Sucrose Effects on the Pancreas
Pancreatic tissues from sucrose-fed rats showed hyalinization with focal inflammatory infiltrates (Fig 4). Macrophage infiltration was greater in the pancreatic interstitium of sucrose fed rats compared to starch fed rats (4.8% vs 1.6% interstitial area, p<0.0006). In contrast, infiltration of macrophages within the islets were not different between groups, although they tended to be greater in sucrose fed rats (5.2% vs 2.6% islet area, p<0.08). These findings were accompanied by an increase in whole pancreas mRNA for chemokine (C-X-C motif) ligand 1 (KC or CXCL-1), monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) (Fig 4). There was also significantly less insulin staining in the islets, consistent with the lower insulin levels and higher glucose levels observed in the blood in the sucrose fed rats (Figure 5 and Table 2).
Figure 4. Islet Injury and Inflammation.
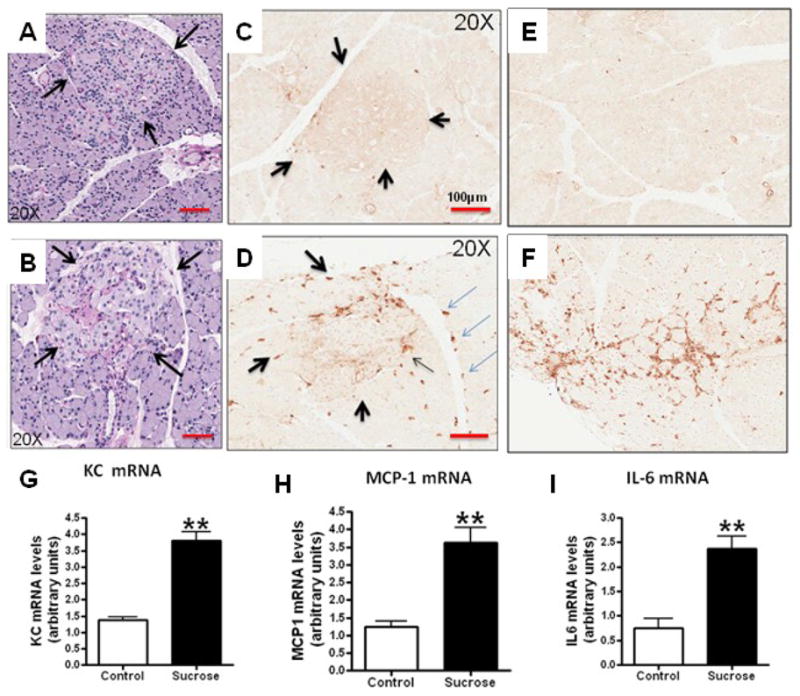
PAS-stained pancreas shows that islets from Starch-fed control rats (identified by arrows) appear normal (A, 20x). In contrast, islets from sucrose-fed rats show focal hyalinization (B, 20x). Whereas macrophages (ED-1 staining) were infrequent in islets (C, 20x) and pancreatic interstitium (E, 10x) of starch fed rats, macrophages were present in both the islets (D, arrows) in venous endothelium (D, light blue arrows) and the interstitium (F, 10x) in sucrose-fed rats). These changes were associated with an increase in KC mRNA, MCP-1 mRNA and IL-6 mRNA in whole pancreas RNA extracts from sucrose fed rats compare to starch fed controls (Figures G–I). *p<0.05, **p<0.01
Figure 5. Decreased Insulin Staining and Expression of Inflammatory Mediators in Sucrose-Fed Rats.
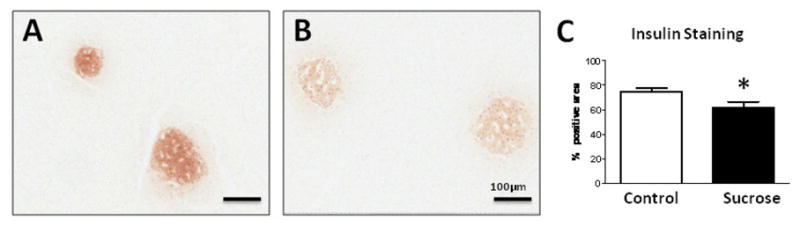
In contrast to starch-fed rats (A), sucrose-fed rats had decreased insulin staining of islets by both intensity and area (B). Quantification documented decreased insulin staining area (C). Western blotting of whole pancreatic extracts for insulin….*p<0.05, **p<0.01 10x
Potential Involvement of Uric acid in Islet Inflammation
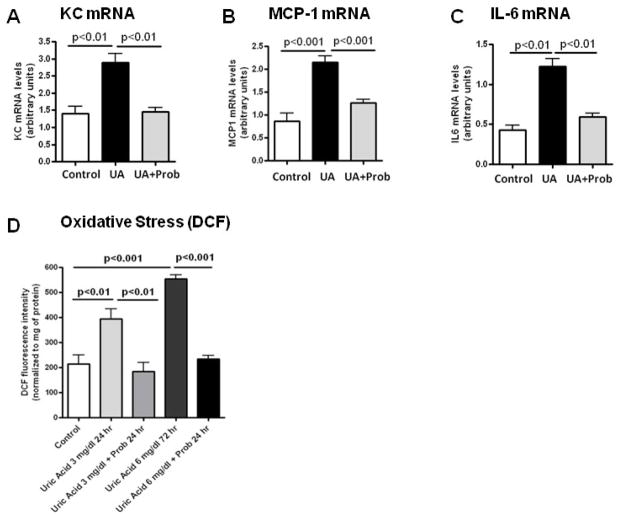
Uric acid is generated during sucrose and fructose metabolism due to the breakdown of adenine nucleotides and the stimulation of AMP deaminase [20]. Uric acid has been shown to enter cells via specific transporters such as URAT-1 where it can induce proinflammatory and prooxidative effects [23–24]. Interestingly, URAT1 expression was increased in the islets and pancreatic blood vessels of sucrose–fed rats by immunostaining (Fig 6). Western blotting confirmed an increase in URAT1 protein in whole pancreas. In addition, incubation of RIN-m5F cells with uric acid resulted in a stimulation of several inflammatory mediators, including MCP-1, KC and IL-6 mRNA, as well as intracellular oxidative stress as determined by increased intensity of the fluorescence probe DCF. Uric acid dependent effects could be inhibited by probenecid, an organic anion transport inhibitor that has been shown to blocks URAT-1 mediated uptake of uric acid in proximal tubular cells [25] (Fig 7).
Figure 6. URAT1 Expression in Pancreatic Islets.
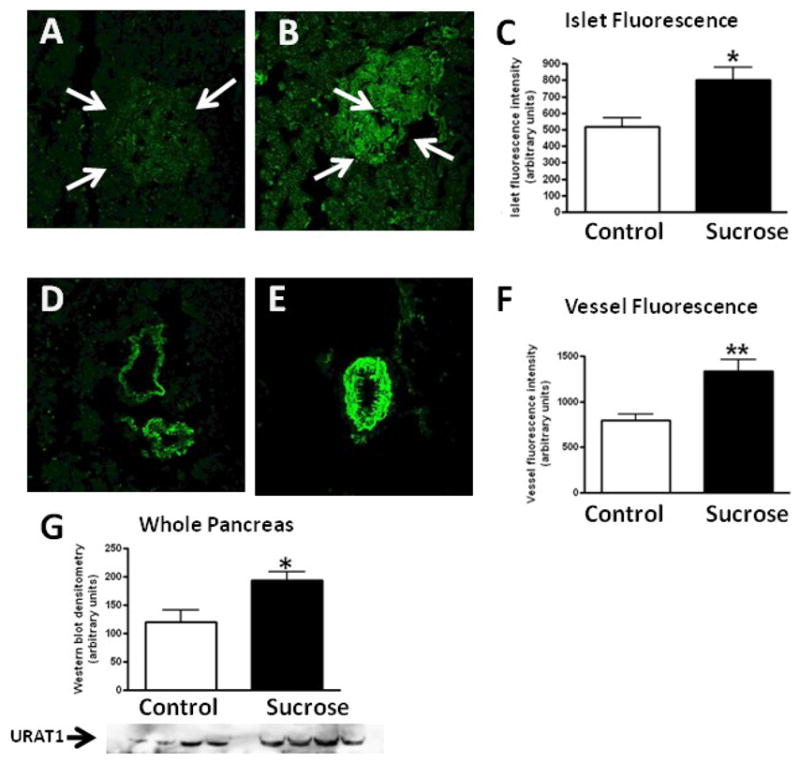
Minimal URAT-1 was expressed by islets in starch fed rats by immunofluorsescence (highlighted by arrows, A) in contrast to sucrose-fed rats (B). Blood vessels also showed minimal URAT-1 staining in starch-fed (D) in contrast to sucrose-fed rats (E). Quantitative immunofluorescence for URAT-1 staining in the islets and blood vessels are shown in C and F. Total URAT-1 protein in whole pancreas by Western blotting showed an approximately 30% increase in URAT-1 in sucrose-fed rats (G). The relatively small increase in URAT-1 protein by Western despite the marked difference in immunostaining is likely because the former is of whole pancreatic extracts that includes the acinar tissue. *p<0.05. 20x.
Figure 7. Effect of Uric acid on Rat Pancreatic Islet Cells.
Pancreatic RIN-m5F cells were exposed for 48 hours to varying concentrations of uric acid in the presence or absence of the URAT-1 inhibitor probenecid (1 mM). Uric acid (UA, 6 mg/dl) significantly increased the mRNA expression of the inflammatory markers KC (Fig A), MCP-1 (Fig B) and IL-6 (Fig C). Uric acid ( 3 and 6 mg/dl) also stimulated oxidative stress, as assessed by oxidized DCF fluorescence (Fig D). that the effects of uric acid to induce cytokine expression and oxidative stress was blocked by probenecid (2 mM) (Figs A–D)
Discussion
While high concentrations of fructose are known to induce metabolic syndrome in male Sprague Dawley rats, the studies have been criticized as being nonphysiologic. In addition, studies using pure fructose have been criticized since they do not mimic dietary habits in humans in which most fructose is from added sugars that also contain glucose either as a disaccharide (sucrose) or as a mixture of free monosaccharides (high fructose corn syrup). Furthermore, there is significant debate over whether the effects of fructose (or of added sugars in general) induce features of metabolic syndrome independent of excessive energy intake. The purpose of this study was therefore to determine if metabolic syndrome can be induced in rats by administering a sucrose-based diet in which the fructose content is substantially less than classic models, and whether the effects of sucrose are independent of excessive energy intake. Studies were performed in older male Sprague Dawley breeder rats, which are known to develop features of metabolic syndrome as they age [12–13]. Hence, these studies address whether sucrose diets can accelerate metabolic syndrome and islet abnormalities as opposed to causing these effects de novo.
Rats were administered 40% sucrose or starch diets for 4 months. The diets were identical in all nutrients except that sucrose was replaced with starch, and all rats were pair fed to assure identical caloric intake. We also mildly diet restricted all rats to assure there be no concern that intake was not excessive. Furthermore, this approach might simulate individuals who calorically restrict as an attempt to diet but who may not reduce their sugar intake. The first new finding was that the sucrose-based diet induced upregulation of Glut5 and fructokinase in the jejunum and jejunum and liver, respectively. Fructose absorption is known to vary dramatically in individuals, and this may be due to variable expression of Glut5 [26]. The observation that a sucrose-based diet containing 20% fructose can upregulate the Glut5 transporter suggests that diets high in added sugars may increase the absorption of fructose and hence its metabolic effects. These data are consistent with our observation that fructokinase mRNA expression is increased in subjects with nonalcoholic fatty liver disease (NAFLD) who are known to be ingesting high levels of fructose-containing beverages [27]. This could provide a potential explanation for why the effect of fructose on insulin resistance and serum triglycerides is greater in overweight or hyperinsulinemic individuals [28–29].
The second new finding was that a sucrose based diet containing 20% fructose could accelerate the development of metabolic syndrome and cause fatty liver. Previously most studies in rats have evaluated the effects of high concentrations of fructose on metabolic syndrome, and/or have not pair fed rats given control diets to determine if the effects are independent of energy intake [30–31]. Our studies show that even when diets are restricted to 90% of normal diet, that features of metabolic syndrome can be accelerated in the male Sprague Dawley rat. The most impressive finding related to the effects on the liver, where fatty liver developed only in the sucrose fed rats.
A third finding was that sucrose intake could increase blood pressure during the immediate postprandial period. Studies in rats have shown that 24 hour blood pressure is not increased by telemetry with diets high in fructose [32]. However, a recent study by Brown et al reported that the administration of fructose, but not glucose, will acutely raise blood pressure in humans [33]. Studies in mice have also confirmed that fructose raises systemic blood pressure by intraaortic telemetry during feeding [34]. The mechanism may relate to the rapid rise in both intracellular and serum uric acid that occurs with fructose ingestion [35–36]. Increasing serum uric acid has been found to raise blood pressure in animals [37]. Hyperuricemia is also associated with hypertension in humans and in pilot studies the lowering of uric acid can reduce blood pressure [38–39]. Although short term trials have generally not shown an effect of fructose on blood pressure in humans, a significant increase in blood pressure when large amounts of fructose was shown in the Menorca study [40]. Furthermore, in this study the rise in blood pressure was associated with an increase in fasting uric acid levels and both the rise in uric acid and blood pressure were prevented in the subjects randomized to allopurinol [40]. The chronic ingestion of fructose-containing drinks is also associated both with an elevation of serum uric acid [41] and with the development of hypertension independent of body mass index [36, 42]. Furthermore, epidemiology studies suggest that reducing sugar-containing soft drinks results in a reduction in blood pressure independent of weight change [43].
Finally, this study documented that sucrose could induce pancreatic inflammation. Specifically, sucrose fed rats showed evidence for mild islet injury associated with a reduction in insulin by immunostaining and with the development of type 2 diabetes. Macrophage infiltration was increased in the interstitium of the pancreas and tended to be higher in the islets, and this was associated with increased mRNA expression in whole pancreatic RNA for a variety of inflammatory mediators including MCP-1. While there are likely many mechanisms possible to account for these findings, we performed pilot studies to evaluate the role of uric acid. The rationale is based on the fact that the metabolism of fructose in the liver results in the stimulation of AMP deaminase with the production of uric acid intracellularly which then results in a rise in serum uric acid. While serum uric acid is most elevated during fructose ingestion, we also observed higher levels during fasting at week 4 (Table 2). In turn, uric acid has been shown to enter a variety of cells via organic anion transporters such as URAT-1 [23, 44] where it can induce proinflammatory and prooxidative effects [45–50]. Uric acid has also been found to independently predict the development of insulin resistance and type 2 diabetes by meta-analysis [51]. Of particular interest are reports by Wexler [52] and Wexler and Greenberg [53] that chronic hyperuricemia induced by a uricase inhibitor can induce hypertension, hypertriglyceridemia, fatty liver and diabetes in the male Sprague Dawley rat. Of interest, the ability to induce these changes correlated with the serum uric acid levels, although proof that this was due to uric acid by studies using uric acid-lowering therapy were not performed [52–53]. Furthermore, in one of these studies a comparison of gender and breeder status was performed, and the male breeder rats showed the greatest elevations in uric acid and the most severe metabolic changes [53].
In turn, we found that URAT1 expression was induced in the islets of sucrose-fed rats and that uric acid could induce proinflammatory and prooxidative effects in islet cells that could be prevented by incubation with the organic anion transport inhibitor, probenecid. Others have also reported that fructose-based diets can induce β cell loss in a genetic model of insulin resistance [9–10] as well as in normal Wistar rats [11]. Recently Cummings et al also reported that a diet of 10% fructose could accelerate the development of type 2 diabetes in a specific diabetes prone rat in association with a reduction in islet cells [6]. There is also a report that soft drink ingestion correlates with evidence for β cell dysfunction in Hispanic adolescents [54]. Collectively, these data support the possibility that fructose containing sugars may increase the risk for diabetes not only by causing insulin resistance but by direct effects on the islets themselves and that one possible mechanism may involve the ability of fructose to raise serum uric acid levels.
This study has several limitations, including small sample size and potential individual variability in rate of food intake. We also used male Sprague Dawley breeder rats, which are known to be prone to develop metabolic syndrome and pancreatic islet changes as they age [12–13]. Fructose is also known to be more likely to induce metabolic changes in older male rats [55–57] and in older male humans [58–59]. Hence, our studies may or may not be relevant to younger or female animals. We are currently studying potential mechanisms to account for the gender and age based differences in the metabolic responses to sucrose and/or fructose with a hypothesis that this may relate to the degree of intracellular and extracellular uric acid generation.
In conclusion, sucrose is not simply an energy source that may have a role in obesity, but rather has specific metabolic effects that favor the development of fat accumulation and insulin resistance independent of excessive energy intake. These observations are consistent with a recent meta analysis has found that the intake of sugar sweetened beverages increases the risk for metabolic syndrome and diabetes in humans [8]. Further studies investigating the potential mechanisms by which sucrose may lead to these metabolic alterations are needed.
Acknowledgments
Supported by NIH grants HL-68607 and startup funds at the University of Colorado (RJJ).
Abbreviations
- ACL
ATP citrate lyase
- AMPD2
adenosine monophosphate deaminase-2
- BP
blood pressure
- KC or CXCL-1
Chemokine (C-X-C motif) ligand 1
- DCF
Fluorescent probe carboxy H2-dichlorodihydrofluorescein
- ECoAH1
Enoyl CoA hydratase-1
- FAS
Fatty acid synthase
- IL-6
Interleukin-6
- KHK-C
Fructokinase C
- MCP-1
Monocyte chemoattractant protein-1
- PAS
Periodic acid Schiff
Footnotes
Disclaimers and Conflict of Interest
Dr R Johnson, Dr Nakagawa, and Dr Lanaspa have patent applications related to lowering uric acid or blocking fructose metabolism in the treatment of metabolic syndrome. Dr Johnson also has a book, the Sugar Fix (Rodale, 2008; and Simon and Schuster, 2009) that discusses the potential role of fructose in the obesity epidemic.
Author Contributions
Study design: CRJ, MAL, CJR, TN, LGS, DJ, RJJ; Performance of Study: CRJ, MAL, CJR, LGS, AAH ; Data analysis; CRJ, MAL, CJR, AAH; KM; Wrote, reviewed and edited manuscript: CRJ, MAL, TN, LGS, DJ, KT, MM, YYS, RJJ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutrition reviews. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 2.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 3.White JS. No unique role for fructose sweeteners in obesity or cardiorenal disease. The American journal of clinical nutrition. 2008;87:1062–1063. doi: 10.1093/ajcn/87.4.1062. author reply 1063–1066. [DOI] [PubMed] [Google Scholar]
- 4.Jones JM. Dietary sweeteners containing fructose: overview of a workshop on the state of the science. The Journal of nutrition. 2009;139:1210S–1213S. doi: 10.3945/jn.108.097972. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120:1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 6.Cummings BP, Stanhope KL, Graham JL, et al. Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: amelioration by the antioxidant, alpha-lipoic acid. American journal of physiology. 2010;298:R1343–1350. doi: 10.1152/ajpregu.00468.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 8.Malik VS, Popkin BM, Bray GA, Despres JP, Willett W. Sugar sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A Meta-analysis. Diabetes care. 2010 doi: 10.2337/dc10-1079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama M, Wada R, Sakuraba H, Mizukami H, Yagihashi S. Accelerated loss of islet beta cells in sucrose-fed Goto-Kakizaki rats, a genetic model of non-insulin-dependent diabetes mellitus. Am J Pathol. 1998;153:537–545. doi: 10.1016/s0002-9440(10)65596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizukami H, Wada R, Koyama M, et al. Augmented beta cell loss and mitochondrial abnormalities in sucrose-fed GK rats. Virchows Arch. 2008;452:383–392. doi: 10.1007/s00428-007-0508-2. [DOI] [PubMed] [Google Scholar]
- 11.Maiztegui B, Borelli MI, Raschia MA, Del Zotto H, Gagliardino JJ. Islet adaptive changes to fructose-induced insulin resistance: beta-cell mass, glucokinase, glucose metabolism, and insulin secretion. J Endocrinol. 2009;200:139–149. doi: 10.1677/JOE-08-0386. [DOI] [PubMed] [Google Scholar]
- 12.Dillberger JE. Age-related pancreatic islet changes in Sprague-Dawley rats. Toxicol Pathol. 1994;22:48–55. doi: 10.1177/019262339402200107. [DOI] [PubMed] [Google Scholar]
- 13.Wexler BC, Greenberg BP. Pathophysiological differences between paired and communal breeding of male and female Sprague-Dawley rats. Circulation research. 1978;42 :126–135. doi: 10.1161/01.res.42.1.126. [DOI] [PubMed] [Google Scholar]
- 14.Glushakova O, Kosugi T, Roncal C, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol. 2008;19:1712–1720. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanaspa MA, Almeida NE, Andres-Hernando A, Rivard CJ, Capasso JM, Berl T. The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13672–13677. doi: 10.1073/pnas.0702752104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korieh A, Crouzoulon G. Dietary regulation of fructose metabolism in the intestine and in the liver of the rat. Duration of the effects of a high fructose diet after the return to the standard diet. Arch Int Physiol Biochim Biophys. 1991;99:455–460. [PubMed] [Google Scholar]
- 17.Weiser MM, Quill H, Isselbacher KJ. Effects of diet on rat intestinal soluble hexokinase and fructokinase activities. The American journal of physiology. 1971;221:844–849. doi: 10.1152/ajplegacy.1971.221.3.844. [DOI] [PubMed] [Google Scholar]
- 18.Burant CF, Saxena M. Rapid reversible substrate regulation of fructose transporter expression in rat small intestine and kidney. The American journal of physiology. 1994;267:G71–79. doi: 10.1152/ajpgi.1994.267.1.G71. [DOI] [PubMed] [Google Scholar]
- 19.Crouzoulon G, Dandrifosse G. Dietary regulation of fructose metabolism in the intestine and in the liver of the rat: time response and effect of cycloheximide. Int J Biochem. 1979;10:439–447. doi: 10.1016/0020-711x(79)90069-7. [DOI] [PubMed] [Google Scholar]
- 20.van den Berghe G, Bronfman M, Vanneste R, Hers HG. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. The Biochemical journal. 1977;162:601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. The American journal of clinical nutrition. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Yamamoto I, Tanaka Y, Ontko JA. Fatty acid metabolism and lipid secretion by perfused livers from rats fed laboratory stock and sucrose-rich diets. J Lipid Res. 1987;28:1156–1165. [PubMed] [Google Scholar]
- 23.Price KL, Sautin YY, Long DA, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 24.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 25.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 26.Van den Berghe G. Fructose: metabolism and short-term effects on carbohydrate and purine metabolic pathways. Progress in biochemical pharmacology. 1986;21:1–32. [PubMed] [Google Scholar]
- 27.Ouyang X, Cirillo P, Sautin Y, et al. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallfrisch J, Ellwood KC, Michaelis OEt, Reiser S, O’Dorisio TM, Prather ES. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. The Journal of nutrition. 1983;113:1819–1826. doi: 10.1093/jn/113.9.1819. [DOI] [PubMed] [Google Scholar]
- 29.Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. The American journal of clinical nutrition. 1983;37 :740–748. doi: 10.1093/ajcn/37.5.740. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman Z, Oron-Herman M, Grozovski M, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–1018. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- 31.Zavaroni I, Sander S, Scott S, Reaven GM. Effect of fructose feeding on insulin secretion and insulin action in the rat. Metabolism: clinical and experimental. 1980;29:970–973. doi: 10.1016/0026-0495(80)90041-4. [DOI] [PubMed] [Google Scholar]
- 32.D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague-Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 33.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. American journal of physiology. 2008;294:R730–737. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 34.Farah V, Elased KM, Chen Y, et al. Nocturnal hypertension in mice consuming a high fructose diet. Auton Neurosci. 2006;130:41–50. doi: 10.1016/j.autneu.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Stirpe F, Della Corte E, Bonetti E, Abbondanza A, Abbati A, De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1311. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. The Journal of pediatrics. 2009;154:807–813. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 38.Feig DI, Soletsky B, Johnson RJ. Effect of Allopurinol on the Blood Pressure of Adolescents with Newly Diagnosed Essential Hypertension. JAMA. 2008;300:922–930. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Pozo SE, Schold J, Nakagawa T, Sanchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–461. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 41.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis and rheumatism. 2007;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 42.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21:1543–1549. doi: 10.1681/ASN.2009111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, Caballero B, Mitchell DC, et al. Reducing Consumption of Sugar-Sweetened Beverages Is Associated With Reduced Blood Pressure. A Prospective Study Among United States Adults. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.911164. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang DH, Han L, Ouyang X, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. American journal of nephrology. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 45.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 46.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. Journal of hypertension. 2010;28 :1234–1242. [PubMed] [Google Scholar]
- 47.Kanellis J, Watanabe S, Li JH, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 48.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. Journal of hypertension. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 49.Cheng TH, Lin JW, Chao HH, et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Cirillo P, Gersch MS, Mu W, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol. 2009;20:545–553. doi: 10.1681/ASN.2008060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes care. 2009;32:1737–1742. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wexler BC. Allantoxanamide-induced myocardial necrosis in Sprague-Dawley vs spontaneously hypertensive rats. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY. 1982;170:476–485. doi: 10.3181/00379727-170-41462. [DOI] [PubMed] [Google Scholar]
- 53.Wexler BC, Greenberg BP. Effect of increased serum urate levels on virgin rats with no arteriosclerosis versus breeder rats with preexistent arteriosclerosis. Metabolism: clinical and experimental. 1977;26:1309–1320. doi: 10.1016/0026-0495(77)90027-0. [DOI] [PubMed] [Google Scholar]
- 54.Davis JN, Ventura EE, Weigensberg MJ, et al. The relation of sugar intake to beta cell function in overweight Latino children. The American journal of clinical nutrition. 2005;82:1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009 doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 56.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283:H2478–2484. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 57.Vrana A, Kazdova L, Dobesova Z, et al. Triglyceridemia, glucoregulation, and blood pressure in various rat strains. Effects of dietary carbohydrates. Ann N Y Acad Sci. 1993;683:57–68. doi: 10.1111/j.1749-6632.1993.tb35692.x. [DOI] [PubMed] [Google Scholar]
- 58.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. The American journal of clinical nutrition. 2000;72:1128–1134. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 59.Couchepin C, Le KA, Bortolotti M, et al. Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes care. 2008;31:1254–1256. doi: 10.2337/dc07-2001. [DOI] [PubMed] [Google Scholar]