FIGURE 1.
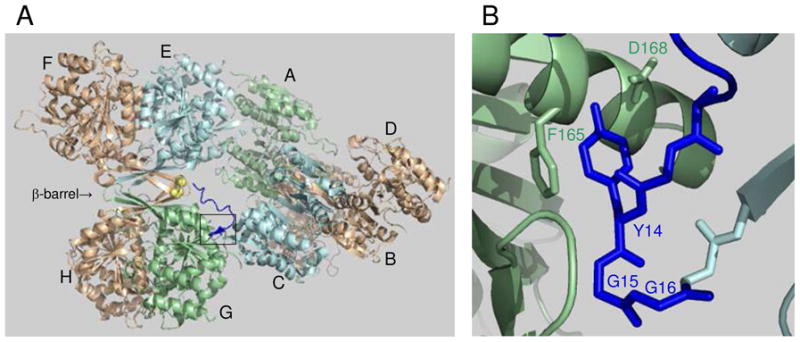
Structure of octameric yeast IDH and interactions at the tetramer interface. (A) Organization of IDH1/IDH2 heterodimers in the IDH octamer (PDB code 3BLV). Catalytic IDH2 subunits shown in light gold are located on the exterior of the octamer, with the regulatory IDH1 subunit in each heterodimer located on the interior of the octamer. Spatially equivalent IDH1 subunits are shown in light blue (C and E) or in light green (A and G). The β-barrel structure at the interface of heterodimers in the EF/GH tetramer is indicated. The amino terminus of IDH1 subunit C (shown in dark blue) extends to interact with the IDH2 Cys-150 residues (shown in yellow) located in the β-barrel structure of the other tetramer. The boxed area indicates the major site of interactions between IDH1 subunits C and G in the tetrameric interface. (B) Detail of the boxed area showing key residues in the tetrameric interface.
