Abstract
Objective:
Various studies have shown the effectiveness of risperidone and fluoxetine in the management of behavioral problems in autism.
Aim:
The purpose of this study was to compare these two drugs in the management of behavioral problems in autism.
Materials and Methods:
Forty children with autism were divided into 2 groups in a 16-week open trial that compared these two drugs. Parents rated the children using the Aberrant Behavior Checklist (ABC) and the Conners′ Parent Rating Scale – Revised (CPRS-R). The author rated the children using the Children's Psychiatric Rating Scale and Clinical Global Impression (CGI) Scale.
Results:
The risperidone group showed significant improvement in areas like irritability and hyperactivity, while the fluoxetine group showed significant improvement in speech deviance, social withdrawal and stereotypy. When the two drugs were compared, fluoxetine showed greater improvement in stereotypy, while both drugs showed improvement on the general autism scale; and on anger, hyperactivity and irritability scales.
Conclusions:
In this open trial, both drugs were well tolerated and appeared to be beneficial in the treatment of common behavioral problems in children with autism. Further controlled and double-blind studies in larger samples are warranted.
Keywords: Autistic disorder, fluoxetine, risperidone
INTRODUCTION
There are currently no treatments available that are specific for autism. Diverse pharmacological agents have been used in children with autistic disorder — few in trials and many reported in anecdotal case reports. Most treatments are aimed at reducing associated behaviors like aggression, agitation, inattention and hyperactivity.[1] The pharmacotherapy of autism is focused on primary emotional and behavioral target symptoms and matching these targets with medications that are likely to be helpful.[2,3]
Risperidone is an atypical antipsychotic drug that belongs to the benzisoxazole derivative class. Its actions are mediated by antagonism of the dopamine type 2 (D2) and serotonin type 2 (5HT2) receptors. It has fewer extrapyramidal symptoms as side effects compared to typical antipsychotics, although reports of tardive dyskinesia, hepatotoxicity and weight gain exist.[4–6] There are a number of reports that suggest modest efficacy of risperidone in the management of behavioral symptoms in children with autistic disorder.[7–11]
Work specifically examining the association between serotonergic regulation and autism dates back to 1961, when a study reported elevated whole blood levels of serotonin in autistic individuals.[12] Potent serotonin-transporter inhibitors are often used to address symptoms like compulsions and repetitive behavior, obsessions, anxiety, irritability and depression. These medications are useful when symptoms like insistence on routine are present and aggression in response to interruption of routines manifests as seen in autistic disorder.[13,14]
Selective serotonin reuptake inhibitors (SSRIs) in open-label investigations have been found to be useful in children with pervasive developmental disorders (PDDs). Favorable responses to nearly all drugs in this group have been noted.[15–21] Common side effects associated with these drugs are decreased appetite, motor restlessness, irritability and insomnia.[22] Larger number of studies and reports are present with fluoxetine than with other drugs. Due to a perceived sensitivity to these agents in those with autism, it is hypothesized that starting at a low dose and titrating slowly upward would prove superior in terms of outcome when using these drugs in autistic children.[23]
To the best of our knowledge, this study is the first open trial that compares these two agents in the management of autistic disorder.
Aim of the study
The aim of the study was to compare the efficacy and safety of risperidone and fluoxetine in children and adolescents with autistic disorder/ autism.
MATERIALS AND METHODS
Forty children with autism and behavioral problems between the ages of 5 and 16 years were part of the study. The diagnosis of autism was made using the Autism Diagnostic Interview – Revised (ADI-R)[24] and clinical observation. All participants met the DSM-IV and ADI-R criteria for autism.[25] Twenty-nine subjects had comorbid mild-to-moderate mental retardation. Children and adolescents with seizure disorder, cardiac disorder, previous exposure to either of the drugs in the study and severe mental retardation were excluded from the study. All the children were required to be off any psychotropic medication for at least 4 weeks prior to the start of the study. All these subjects had shown minimal or no response to the previous medications. The subjects were previously on psychotropic medication like Citalopram, Escitalopram, Venlafaxine, Methylphenidate, Haloperidol, Olanzapine and Piracetam.[26] The parents of all subjects provided a written consent for participation in the study after being informed about all the details of the study.
Dosage of medication
The study was a prospective open trial. Risperidone was administered at a dose of 0.5 mg per day for 1 week and 0.5 mg twice daily after a week. The daily dose was increased as tolerated and clinically indicated in 0.5-mg increments per week. The maximum dose reached was 2-3 mg per day. Dose reductions were also permitted to manage adverse effects. Fluoxetine was administered at a dose of 10 mg per day (morning) for 1 week and 10 mg twice daily (morning and afternoon) after a week. The daily dose was increased as tolerated and clinically indicated in 10-mg increments per week. The maximum dose reached was 20-40 mg per day. The children did not receive any other medication during the study. The total duration of the study was 4 months (16 weeks).
Scales used for behavior rating
The children were seen every week for the first 4 weeks and every 4 weeks thereafter. Behavior ratings were completed at the start and at the end of the study. Parent-rated outcome measures were used in the study, which included the Aberrant Behavior Checklist (ABC)[27] and the long form of the Conners’ Parent Rating Scale – Revised.[28] Clinician rating scales were completed by the same clinician and included the Children's Psychiatric Rating Scale (Fish)[29] and the Clinical Global Impressions (CGI) Scale.[30] Only the 14 items on the Children's Psychiatric Rating Scale that were relevant to children and adolescents with autism were rated.[31]
Safety assessment
Adverse effects were rated every 4 weeks using the Subjective Treatment-Emergent Symptoms Scale.[32] Vital parameters (heart rate and blood pressure), weight and ECG were assessed at baseline and every 4 weeks during the course of the study. All the patients underwent physical examination prior to entry into the study, and routine laboratory investigations were carried out.
Statistical analysis
Efficacy variables included the severity subscale of the CGI; the irritability, social withdrawal, hyperactivity, stereotypy and excessive speech subscales of the ABC; the emotional liability, inattention and hyperactivity scales of the Conners′ Parents Rating Scale – Revised; and the autism, anger, hyperactivity and unusual speech subscales of the Children's Psychiatric Rating Scale. The statistical analysis used for all efficacy and safety variables (weight, heart rate, blood pressure and ECG parameters) was on intent to treat analysis with the last observation being carried forward. Matched-pair t tests were used to assess the change from baseline to the final observation in each variable. Responders were defined as those with a score of 2 (much improved) or better on the improvement subscale of the CGI scale at the end of the study. All statistical analyses were done by a bio-statistician, and P<.05 was considered as significant in all cases.
RESULTS AND DISCUSSION
Out of the 40 patients, 36 completed the trial. From the fluoxetine group, 2 subjects withdrew due to repeated vomiting, which subsided upon discontinuation of the drug. Both withdrew within the first 6 weeks of the study. From the risperidone group, 2 subjects withdrew, one due to worsening of symptoms while one was withdrawn due to lack of follow-up.
The mean final dose of risperidone and fluoxetine was 2.3 ± 0.7 mg and 28.2 ± 8.3 mg per day, respectively.
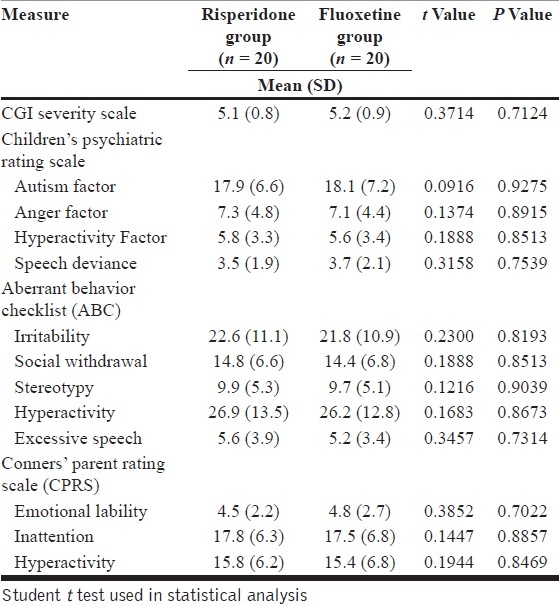
Both groups were well matched in all respects at the start of the study [Table 1].
Table 1.
Baseline scores in the two groups
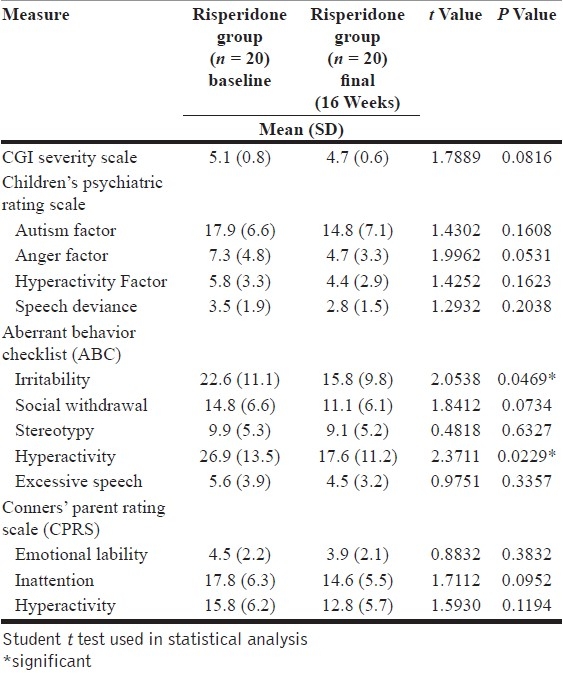
On comparing baseline and final scores for risperidone, it was noted that risperidone showed significantly greater improvement in areas like hyperactivity (P=.0229) and irritability (P=.0469) [Table 2]. Anger and inattention were other areas where improvement was noted, though not statistically significant. Differences on hyperactivity across different scales could probably indicate symptom cluster improvements or differences due to differences in rating methods within different scales. This is in keeping with the mechanism of action and target symptoms in focus when administering risperidone.[7,11]
Table 2.
Baseline vs. final scores in the risperidone group
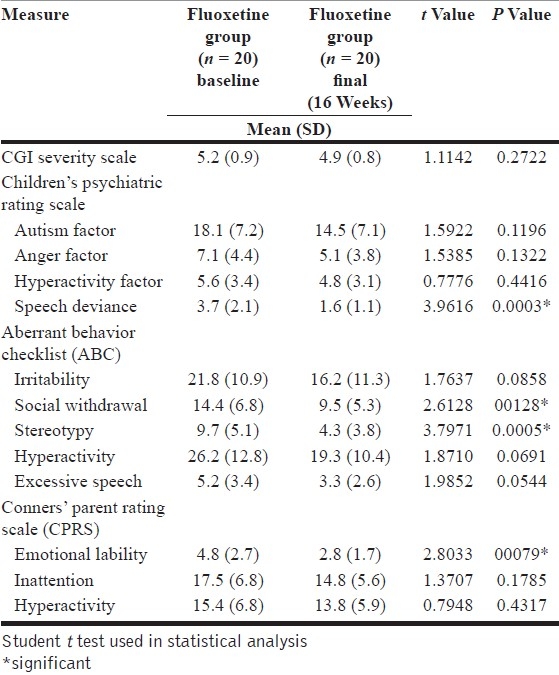
On comparing the baseline and final scores in the fluoxetine group, the autistic children, however, showed significantly greater improvement in areas like stereotypy (P=.0005), emotional liability (P=.0079) and speech deviance (P=.0003). Significant improvement was also noted in areas like social withdrawal (P=.0128), while improvement was noted in areas of excessive speech [Table 3]. This is in keeping with the serotonergic action of the drug and its action on obsessive and repetitive symptoms.[3,14]
Table 3.
Baseline vs. final scores in the fluoxetine group
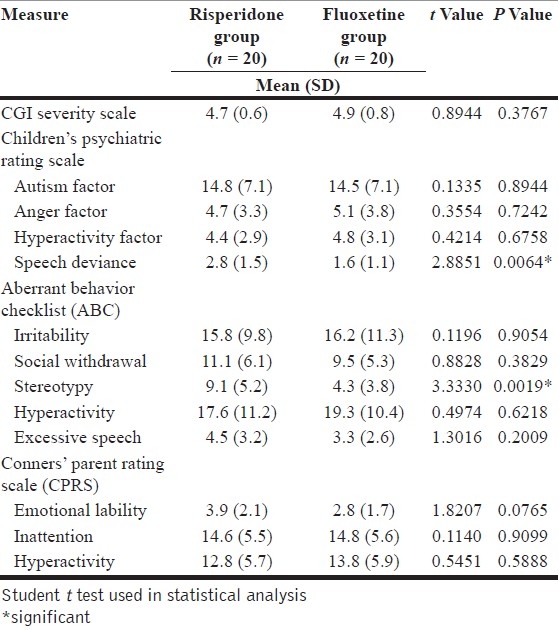
When the final scores of both drugs were compared, fluoxetine showed greater improvement than risperidone in areas of speech deviance (P=.0064) and stereotypy (P=.0019) [Table 4]. Both drugs showed similar improvements on the generalized autism scores of the Children's Psychiatric Rating Scale, hyperactivity, irritability, anger and inattention. The findings are in keeping with some large placebo-controlled trial.[33]
Table 4.
Final scores in the two groups
Risperidone has been known to reduce anger, hyperactivity and aggression in autism, mental retardation, conduct disorder, oppositional defiant disorder, childhood-onset schizophrenia and attention deficit hyperactivity disorder (ADHD).[34] As mentioned earlier, fluoxetine and other SSRIs show greater efficacy in the amelioration of obsessive-spectrum symptoms like verbal and motor perseveration, stereotypy, insistence on routine and the aggression manifested as a result of interruption of routine.[35]
Fluoxetine has documented efficacy in the management of similar symptoms in childhood obsessive compulsive disorder (OCD), Tourette's disorder, ADHD and selective mutism.[36–38]
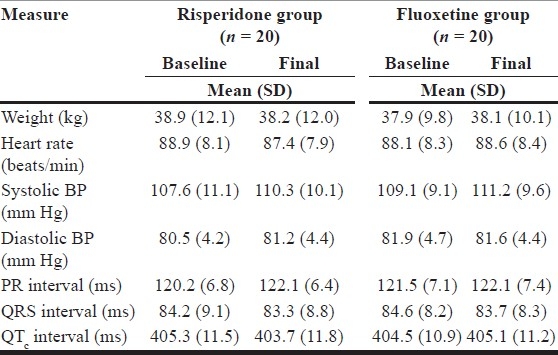
Both drugs showed a good safety profile, with 2 dropouts from the fluoxetine group related to gastrointestinal side effects. There were no significant changes in any clinical measures of safety noted in the study [Table 5].
Table 5.
Scores on safety measures in both groups
In conjunction with other studies, these results provide further evidence suggesting the efficacy of these drugs in children with autism. Since the two drugs are of diverse pharmacological classes and act on different target symptoms in autism, there are differences noted in the improvement with respect to behavioral profiles of children receiving these drugs. Though the results of this study are encouraging, they must be interpreted cautiously in the light of several limitations. The sample was small in size and thus does not provide enough statistical power to support the positive conclusions gained from this data. There may have also been insufficient power to detect treatment-related changes of lesser magnitude than seen here. The open-label nature of this study suggests that there may exist a potential for bias and the possibility that improvement with these drugs may also be seen due to natural fluctuations in the behavioral difficulties seen in autism, placebo response or expectancy effects.[39] This may explain the differences in findings with regard to improvement as noted in the study when compared to double-blind studies involving these two drugs.
Though parent-rated improvements were noted, parent-rated scales are difficult to assess clinically in autism. The present study is a brief one and further studies will be needed to assess the long-term benefits and safety of these drugs to treat behavioral problems in autistic children.[40] This study is a positive step in that regard and may also lead the way for further studies that may combine these drugs to enhance better response in different symptom areas of autism.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Posey DJ, McDougle CJ. Pharmacotherapuetic management of autism. Exp Opin Pharmacother. 2001;2:587–600. doi: 10.1517/14656566.2.4.587. [DOI] [PubMed] [Google Scholar]
- 2.Aman M, Lam KS, Van Bourgondien ME. Medication patterns in autsm: temporal, regional and demographic influences. J Child Adolesc Psychopharmacol. 2005;15:116–26. doi: 10.1089/cap.2005.15.116. [DOI] [PubMed] [Google Scholar]
- 3.Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet. 2003;362:732–4. doi: 10.1016/S0140-6736(03)14236-5. [DOI] [PubMed] [Google Scholar]
- 4.Green WH. Child and Adolescent Psychopharmacology. Lippincott: Williams and Wilkins; 2001. [Google Scholar]
- 5.Martin A, Landau J, Leebens P, Ulizio K, Cicchetti D, Scahill L, Leckman JF. Risperidone associated weight gain in children and adolescents: a retrospective chart review. J Child Adolesc Psychopharmacol. 2000;10:259–68. doi: 10.1089/cap.2000.10.259. [DOI] [PubMed] [Google Scholar]
- 6.Szigethy E, Wiznitzer M, Branicky LA, Maxwell K, Findling RL. Risperidone induced hepatotoxicity in children and adolescents: a chart review study. J Child Adolesc Psychopharmacol. 1999;9:93–8. doi: 10.1089/cap.1999.9.93. [DOI] [PubMed] [Google Scholar]
- 7.Barnard L, Young AH, Pearson J, Geddes J, O’Brien G. A systematic review of the use of atypical antipsychotics in autism. J Psychopharmacol. 2002;16:93–101. doi: 10.1177/026988110201600113. [DOI] [PubMed] [Google Scholar]
- 8.Fisman S, Steele M. Use of risperidone in pervasive developmental disorders: a case series. J Child Adolesc Psychopharmacol. 1996;6:177–90. doi: 10.1089/cap.1996.6.177. [DOI] [PubMed] [Google Scholar]
- 9.Horrigan JP, Barnhill LJ. Risperidone and explosive aggressive autism. J Autism Dev Dis. 1997;27:313–23. doi: 10.1023/a:1025854532079. [DOI] [PubMed] [Google Scholar]
- 10.Simeon JG, Wiggins DM, Williams E. Worldwide use of psychotropic drugs in child and adolescent psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:455–65. doi: 10.1016/0278-5846(95)00026-r. [DOI] [PubMed] [Google Scholar]
- 11.Zuddas A, DiMartino A, Muglia P, Cianchetti C. Long term risperidone treatment for pervasive developmental disorder: efficacy, tolerability and discontinuation. J Child Adolesc Psychopharmacol. 2000;10:79–90. doi: 10.1089/cap.2000.10.79. [DOI] [PubMed] [Google Scholar]
- 12.Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatrics. 1961;58:315–20. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- 13.Brodkin ES, McDougle CJ, Naylor ST, Cohen DJ, Price LH. Clomipramine in adults with pervasive developmental disorders: a prospective open label investigation. J Child Adolesc Psychopharmacol. 1997;7:109–21. doi: 10.1089/cap.1997.7.109. [DOI] [PubMed] [Google Scholar]
- 14.Cook EH, Leventhal BL. The serotonin system in autism. Curr Opin Pediatrics. 1996;8:348–54. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Cook EH, Rowlett R, Jaselskis C, Leventhal BL. Fluoxetine treatment of patients with autism and mental retardation. J Am Acad Child Adolesc Psychiatry. 1992;31:739–45. doi: 10.1097/00004583-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Couturier JL, Nicolson R. A retrospective assessment of Citalopram in children and adolescents with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2002;12:243–8. doi: 10.1089/104454602760386932. [DOI] [PubMed] [Google Scholar]
- 17.DeLong GR, Teague LA, McSwain Karman M. Effects of Fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol. 1998;40:551–62. doi: 10.1111/j.1469-8749.1998.tb15414.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghaziuddin M, Tsai L, Ghaziuddin N. Fluoxetine in autism with depression. J Am Acad Child Adolesc Psychiatry. 1991;30:508–9. doi: 10.1097/00004583-199105000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Hellings JA, Kelley LA, Gabrielli WF, Kilgore E, Shah P. Sertraline response in adults with mental retardation and autistic disorder. J Clin Psychiatry. 1996;57:333–6. [PubMed] [Google Scholar]
- 20.Martin A, Keonig K, Anderson GM. Low dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective open label study. J Autism Dev Dis. 2003;33:77–85. doi: 10.1023/a:1022234605695. [DOI] [PubMed] [Google Scholar]
- 21.Posey DI, Litwiller M, Koburm A, McDougle CJ. Paroxetine in autism. J Am Acad Child Adolesc Psychiatry. 1999;38:111–2. doi: 10.1097/00004583-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Carlson GA, Mick E. Drug induced disinhibition in psychiatrically hospitalized children. J Child Adolesc Psychopharmacol. 2003;13:153–63. doi: 10.1089/104454603322163871. [DOI] [PubMed] [Google Scholar]
- 23.Owley T. The pharmacological treatment of autistic spectrum disorders. CNS Spectrums. 2002;7:663–9. doi: 10.1017/s109285290002215x. [DOI] [PubMed] [Google Scholar]
- 24.Lord C, Rutter M, Le Conteur A. Autism Diagnostic Interview - Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Dis. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 25.Diagnostic and Statistical Manual of Psychiatric Disorders – IV (DSM-IV) American Psychiatric Press; 1994. American Psychiatric Association (APA) [Google Scholar]
- 26.Owley T, Walton RN, Salt JD, Guter SJ, Jr, Winnega M, Leventhal BL, et al. An Open label trial of Escitalopram in Pervasive Developmental Disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:343–8. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- 27.Brown EC, Aman MG, Havercamp SM. Factor analysis and norms for parent ratings on the Abberant Behavior Checklist – Community for young people in special education. Res Dev Disabil. 2002;23:45–60. doi: 10.1016/s0891-4222(01)00091-9. [DOI] [PubMed] [Google Scholar]
- 28.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conner's Parent Ratings Scale (CPRS-R): factor structure, reliability and criterion validity. J Abn Child Psychol. 1998;26:257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 29.Fish B. Children's Psychiatric Rating Scale. Psychopharmacol Bull. 1985;21:753–70. [Google Scholar]
- 30.Guy W. Rockville (Maryland) Department of Health. Education and Welfare; 1976. ECDEU Assessment Manual for Psychopharmacology revised. [Google Scholar]
- 31.Overall JE, Campbell M. Behavioral assessment of psychopathology in children: infantile autism. J Child Psychol. 1998;44:708–16. doi: 10.1002/1097-4679(198809)44:5<708::aid-jclp2270440507>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Campbell M, Palij M. Measurement of side effects including tardive dyskinesia. Psychopharmacol Bull. 1985;21:1063–82. [PubMed] [Google Scholar]
- 33.Research Units on Pediatric Psychopharmacology Autism Network (RUPPN). Risperidone in children with autism and serious behavioral problems. New Eng J Med. 2002;347:314–21. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 34.Armenteros JL, Whitaker AH, Welikson M, Stedge DJ, Gorman I. Risperidone in adolescents with schizophrenia: an open pilot study. J Am Acad Child Adolesc Psychiatry. 1997;36:694–700. doi: 10.1097/00004583-199705000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Aman NG, Singh NN. Psychopharmacology of developmental disabilities. New York: Springer-Verlag; 1988. [Google Scholar]
- 36.Black B, Uhde TW. Treatment of elective mutism with fluoxetine: a double blind placebo controlled study. J Am Acad Child Adolesc Psychiatry. 1994;33:1000–6. doi: 10.1097/00004583-199409000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Gammon GD, Brown TE. Fluoxetine and methylphenidate combination in the treatment of attention deficit disorder in children. J Child Adolesc Psychopharmacol. 1993;3:1–10. doi: 10.1089/cap.1993.3.1. [DOI] [PubMed] [Google Scholar]
- 38.Riddle MA, Hardin MT, King R, Scahill L, Woolston JL. Fluoxetine treatment of children and adolescents with Tourette's and obsessive compulsive disorders: preliminary clinical experience. J Am Acad Child Adolesc Psychiatry. 1990;29:45–8. doi: 10.1097/00004583-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Hardan AY, Handen BL. A retrospective open trial of adjunctive donepezil in children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol. 2002;12:237–41. doi: 10.1089/104454602760386923. [DOI] [PubMed] [Google Scholar]
- 40.Nicolson R, Awad G, Sloman L. An open label trial of Risperidone in young autistic children. J Am Acad Child Adolesc Psychiatry. 1997;37:372–6. doi: 10.1097/00004583-199804000-00014. [DOI] [PubMed] [Google Scholar]


