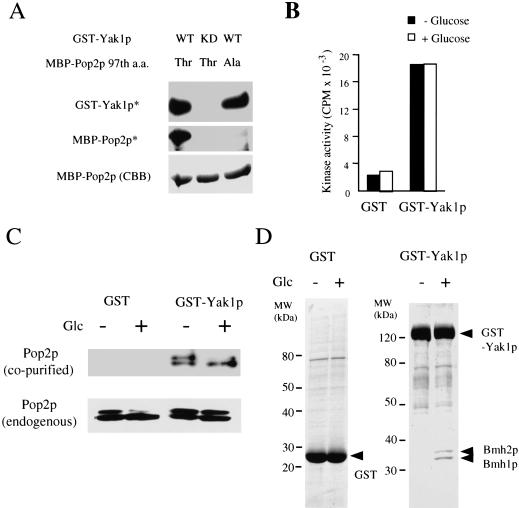
Figure 5.
Analysis of Yak1p using GST-Yak1p fusion protein. (A) GST-Yak1p phosphorylates Thr 97 of full-length Pop2p. Phosphorylation assays were performed as described in the Materials and Methods. (WT) Wild-type Yak1p; (KD, kinase dead) K398R mutant of Yak1p; (Thr) wild-type Pop2p; (Ala) alanine substitution mutant for the 97th threonine of Pop2p; (GST-Yak1p*) autophosphorylation product of GST-Yak1p; (MBP-Pop2p*) phosphorylated MBP-Pop2p; (MBP-Pop2p [CBB]) CBB staining of MBP-Pop2p. (B) In vitro Pop2 peptide kinase activity of GST-Yak1p was not regulated by glucose. The Pop2 peptide phosphorylation activities of GST fusion proteins purified from yeast after treatment with the glucose conditions indicated were determined. (C) Pop2p associates with Yak1p. GST fusion proteins were purified from yeast cells after 10 min incubation in YPD medium (Glc +) or YP (Glc −) and separated by SDS-PAGE, and the proteins were stained with CBB. The Pop2p copurified with the GST fusion proteins was detected using anti-Pop2p specific antibody (Pop2p [co-purified]). The endogenous Pop2p in each yeast cell was detected by Western blotting (Pop2p [endogenous]). (D) Yak1p associates with Bmh1/2p in a glucose-dependent manner. SDS-PAGE image of GST and GST-Yak1p purified in panel C. The amino acid sequences of peptides derived from Yak1p-associated proteins were determined and analyzed as described in the Materials and Methods.