Table 1.
Effects of different groups of endocrine disruptorpesticides and their chemical structures (adapted from [65]).
| Pesticides [66] | Chemical structure | Endocrine Disruptor Effects | Biomonitoring in human samples |
|---|---|---|---|
|
2,4-D (H) M(g/Mol) = 221 pKa = 2.73 logP: 2.81 |
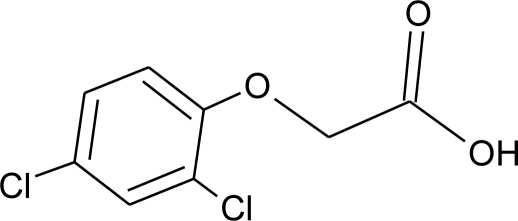 |
Synergistic androgenic effects when combined with testosterone [67] | U: <LOD–598 ng/mL [68] S : 0.07–0.56 μg/g creatinine [69] |
|
Acephate (I) M(g/Mol) = 183.2 pKa = 8.35 logP: −0.85 |
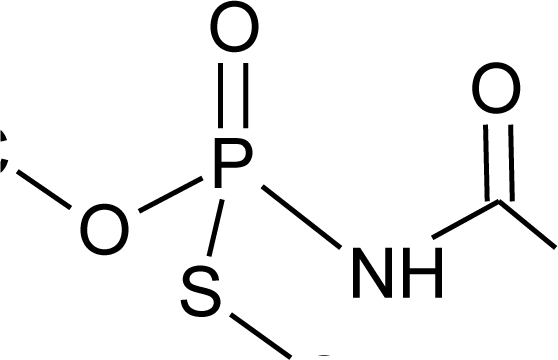 |
Disruption of hormone expression in the hypothalamus [70] | U: <LOD–0.26 ng/mL [68] H.S: 7.2 μg/mL [71] ** |
|
Acetochlor (H) M(g/Mol) = 269.8 pKa = n.a logP: 4.14 |
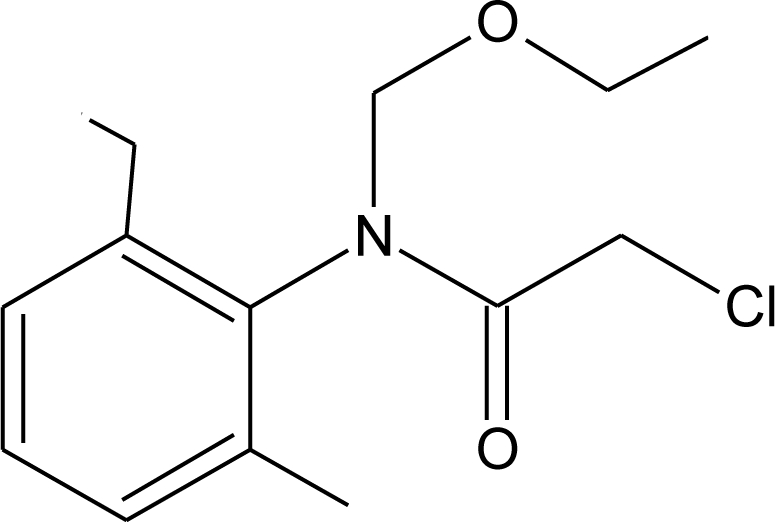 |
Interaction with uterine estrogen receptors, alteration of thyroid hormone dependant gene expression [72,73] | U: <LOD–10.9 ng/mL [68] S: 0.08–0.10 μg/g creatinine [69] |
|
Alachlor (H) M(g/Mol) = 269.8 pKa = 0.62 logP: 3.09 |
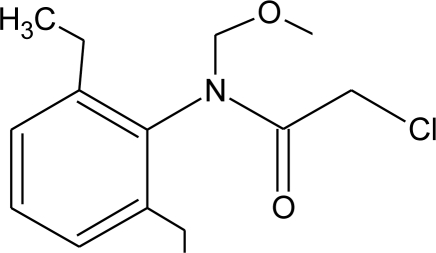 |
Competitive binding to estrogen and progesterone receptors. Interaction with the pregnane X cellular receptor, interfering with the production of enzymes responsible for steroid hormone metabolism [13,74] | U: <LOD–305 ng/mL [68] S: 0.31–0.72 μg/g creatinine [69] |
|
Aldicarb (I) M(g/Mol) = 190.3 pKa = n.a logP: 1.15 |
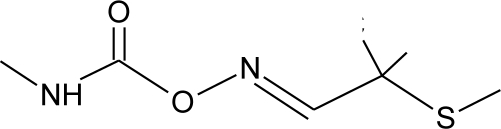 |
Inhibition of 17 beta-estradiol and progesterone activity [13,75] | |
|
Aldrin (I) M(g/Mol) = 364.9 pKa = n.a logP: 6.5 |
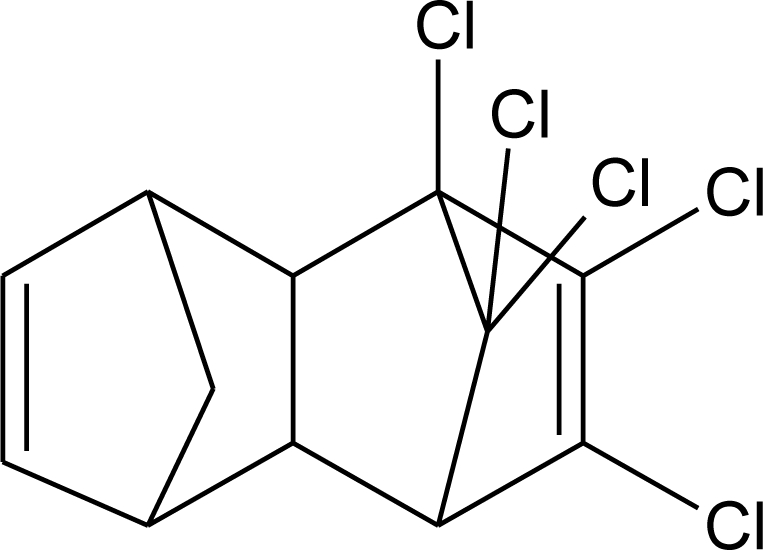 |
Competitive binding to androgen receptors [76] | H.S: 2.17–372 μg/L [77,78] H.M: mean 0.03 mg/L ± 0.03 [79] A.T: 25.6–137.2 ng/g lipid [78] |
|
Atrazine (H) M(g/Mol) = 215.7 PKa = 4.14, 10.7 logP: −0.97 |
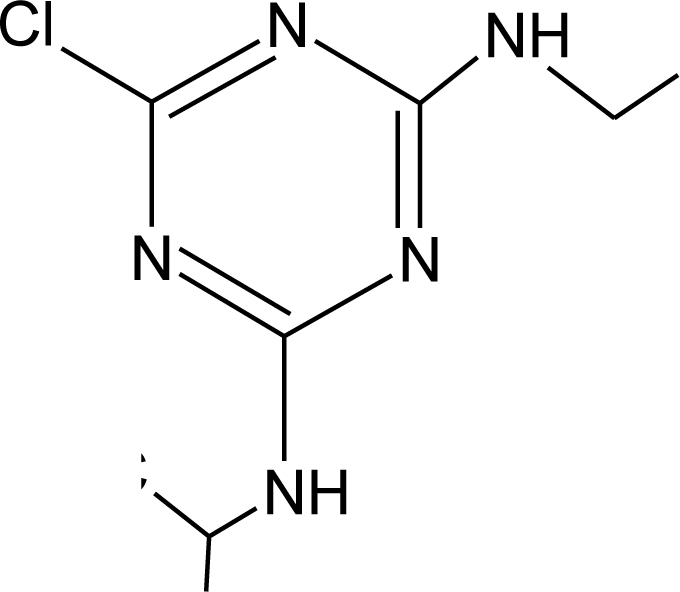 |
Androgen inhibition, weak estrogenic effect. Disruption of the hypothalamic control of lutenising hormone and prolactin levels. Induction of aromatase activity, increase estrogen production. Adrenal glands damages and reduction of steroid hormone metabolism [13,80–83] |
U: <LOD–9.2 ng/mL [68,84] H.S: mean 2 pg/g [76,85] S: 0.07–0.17 μg/g creatinine [69] |
|
Bendiocarb (I) M(g/Mol) = 223.2 pKa = 8.8 logP: 1.7 |
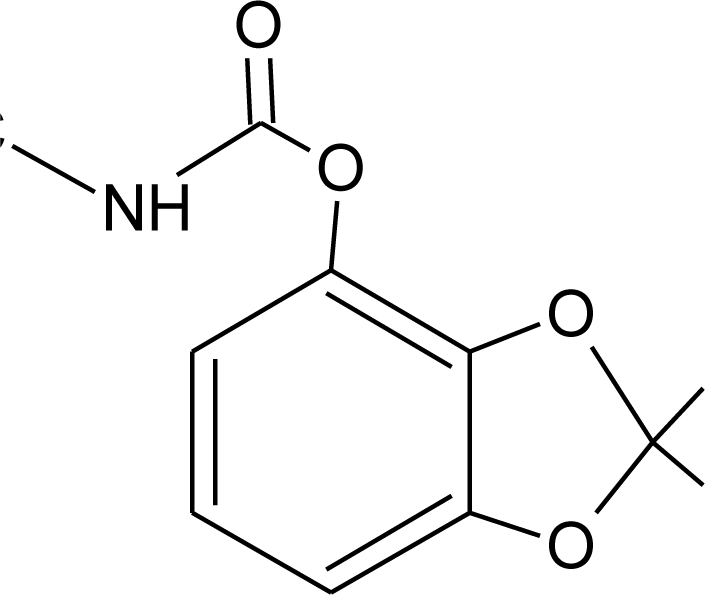 |
Weak estrogen effect [13] | |
|
Benomyl (F) M(g/Mol) = 290.3 pKa = 4.48 logP: 1.4 |
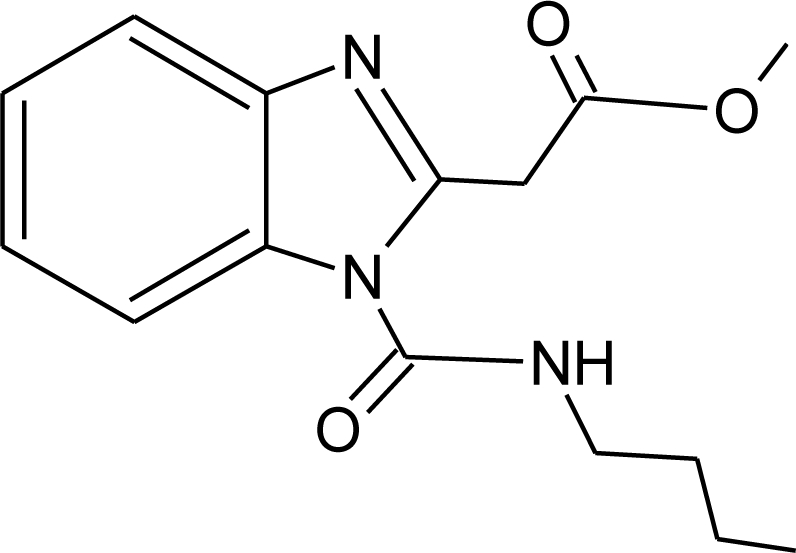 |
Increase of estrogen production and aromatase activity [86] | |
|
Bioallethrin (I) M(g/Mol) = 302.4 pKa = n.a logP: 4.68 |
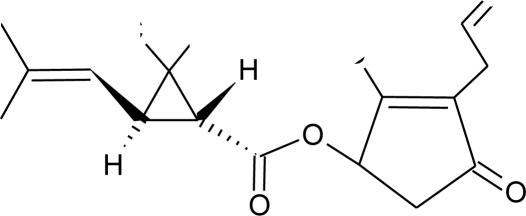 |
Inhibition of estrogen-sensitive cells proliferation [87] | M: 0.61–1.79 μg/mL [88] H: 1.08–2.74 μg/mL [88] |
|
Bitertanol (F) M(g/Mol) = 337.4 pKa = n.a logP: 4.1 |
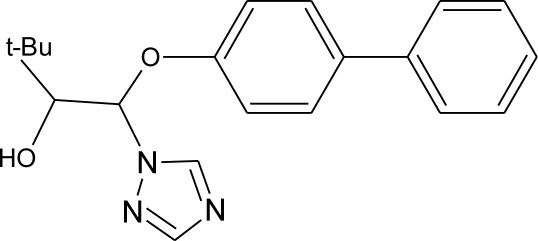 |
Inhibition of aromatase activity, decrease of estrogens production and increase of androgens availability [89] | |
|
Bupirimate (F) M(g/Mol) = 316.4 pKa = 4.4 logP: 3.68 |
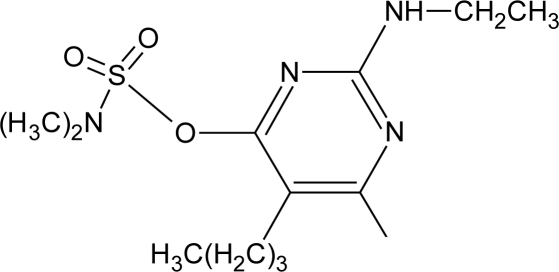 |
Activation of Pregnane X cellular receptor [11] | |
|
Captan (F) M(g/Mol) = 300.6 pKa = n.a logP: 2.5 |
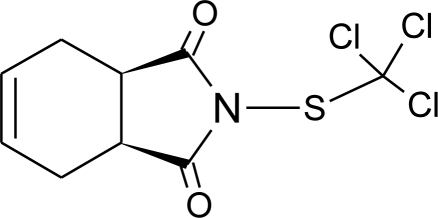 |
Inhibition of estrogen action [90] | |
|
Carbaryl (I) M(g/Mol) = 201.2 pKa = 10.4 logP: 2.36 |
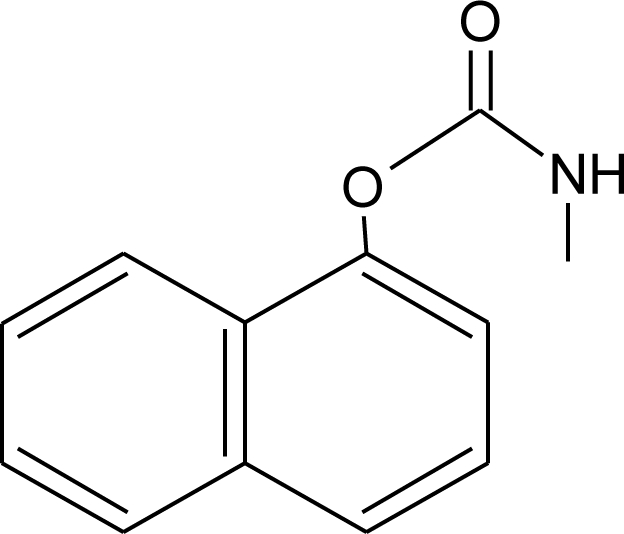 |
Weak estrogen effect [13] | |
|
Carbendazim (F) M(g/Mol) = 191.2 pKa = 4.2 logP: 1.48 |
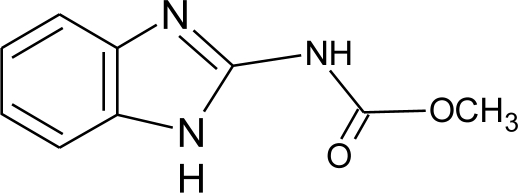 |
Increase of estrogen production and aromatase activity [86] | |
|
Carbofuran (I) M(g/Mol) = 221.2 pKa = n.a logP: 1.8 |
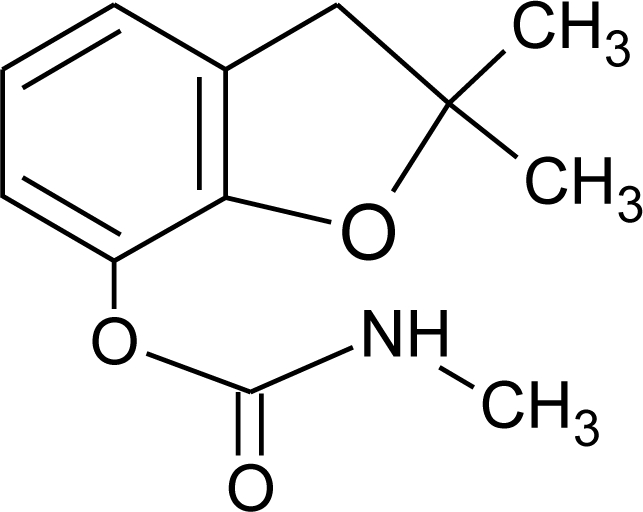 |
Increase of progesterone, cortisol and estradiol level and decrease of testosterone one [91] | M.S: 0.007–17.63 ng/g [92] U.C: 0.007–13.97 ng/g [92] |
|
Chlorothalonil (F) M(g/Mol) = 265.9 pKa = n.a logP: 2.94 |
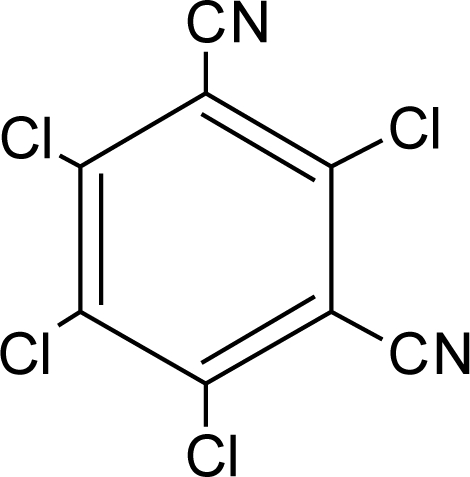 |
Activation of androgen-sensitive cells proliferation [93] | M.S: 0.007–25.31 ng/g [92] U.C: 0.007–25.12 ng/g [92] H.S: mean 6 pg/g [85] |
|
Chlordane (I) M(g/Mol) = 409.8 pKa = n.a logP: 2.78 |
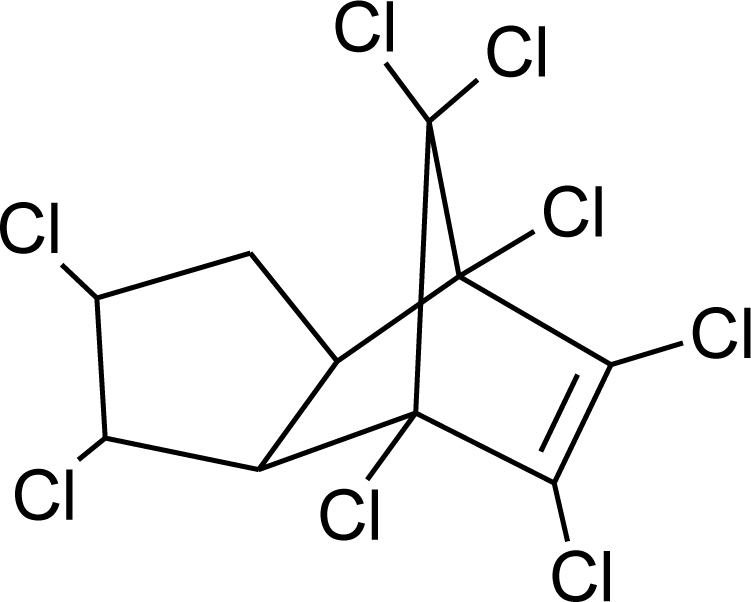 |
Competitive binding to androgen receptors [76] Anti-estrogenic effect, inhibition of estradiol binding [13] | M.P: 0–2.7 ng/g lipid [94] B.S: <LOD–0.9 ng/g lipid [95] H.M: 0.02–437 ng/g lipid [79,96–99] FF: 0.1–0.3 ng/L [100] |
|
Chlordecone (I) M(g/Mol) = 490.6 pKa = n.a logP: 4.5 |
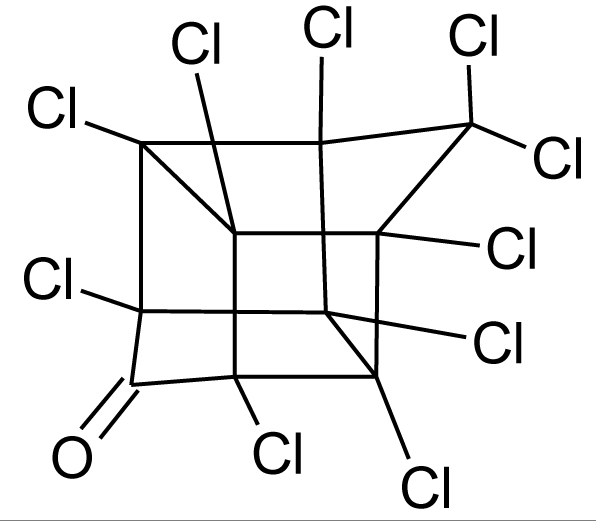 |
Binding to estrogen and androgen receptors [90,101,102] | |
|
Chlorfenviphos (I) M(g/Mol) = 359.6 pKa = n.a logP: 1.36 |
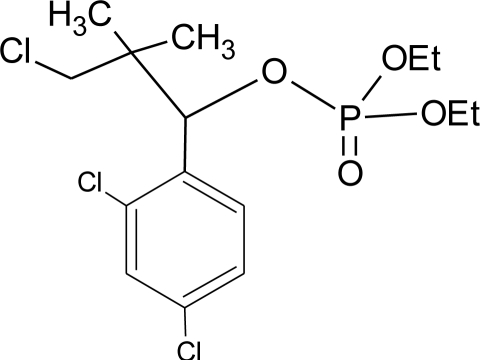 |
Weak estrogen effect [103] | |
|
Chlorpyrifos methyl (I) M(g/Mol) = 322.5 pKa = n.a logP: 4 |
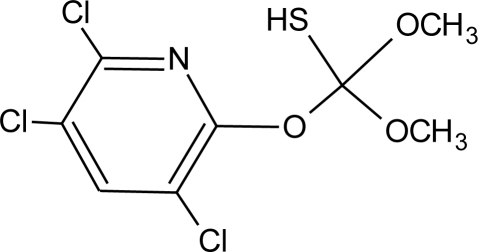 |
Antagonist to androgen activity [104] | U : <LOD–57.7 ng/mL* [68,84] M.S: 0.0007–10.1 ng/g [92] U.C: 0.0007–1.84 ng/g [92] H.S: mean 9 pg/g [85] H: 1.77–2.16 1.83 μg/mL [88] |
|
Cypermethrin (I) M(g/Mol) = 416.3 pKa = n.a logP: 5.3 |
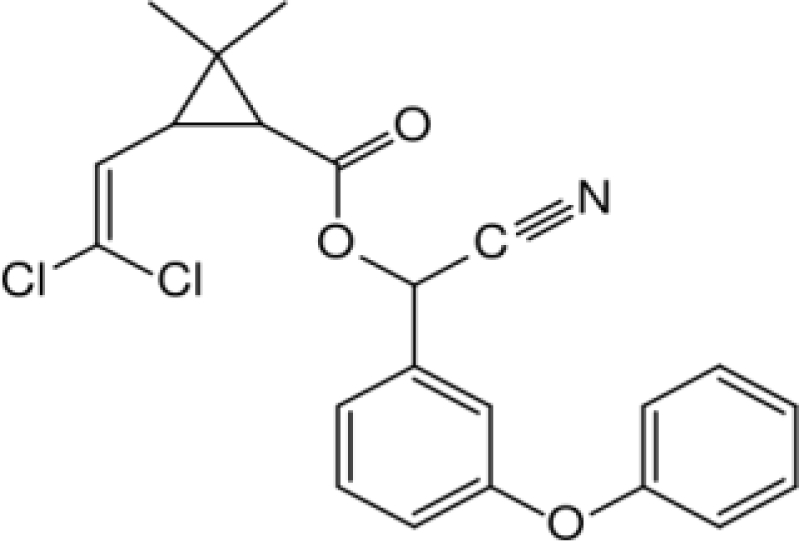 |
Estrogenic effect [105,106] | U: 0.5–100.4 μg/g * [107] M: 1.85–2.43 μg/mL [88] |
|
Cyproconazole (F) M(g/Mol) = 291.8 pKa = n.a logP:3.09 |
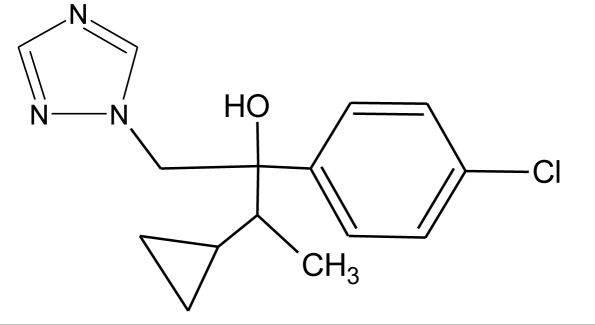 |
Inhibition of aromatase activity, decrease of estrogens production and increase of androgens availability [89] | |
|
DDT and metabolites (I) M(g/Mol) = 354.5 pKa = n.a logP:6.91 |
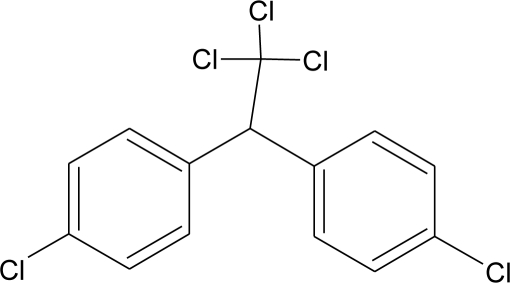 |
Competitive binding to androgen receptors, activation of androgen-sensitive cells proliferation. Stimulation of estrogen receptor production, estrogen receptor agonist and PR antagonist [76,93,108,109] |
M.P: 0.2–3588 ng/g lipid [94] B.S: <LOD–40.9 ng/g lipid [95] HM: 3.9–4700 ng/g lipid [110,96–99,111] A.F: 0.1–0.63 mg/L [112] M: 1.1–2.8 μg/mL [88] H: 0.17–0.65 μg/mL [88,113] M.B: 0–6168 ng/g lipid [110,114] H.S: 12.5–814.9 ng/mL [77] U.C: 189–3296 ng/g lipid [110] |
|
Deltamethrin (I) M(g/Mol) = 505.2 pKa = n.a logP: 4.6 |
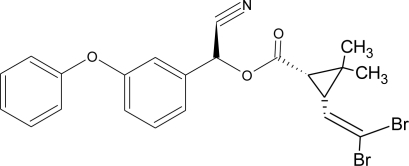 |
Weak estrogenic activity [8] | U: 0.5–57.7 μg/g * [107] |
|
Diazinon (I) M(g/Mol) = 304.4 pKa = 2.6 logP: 3.69 |
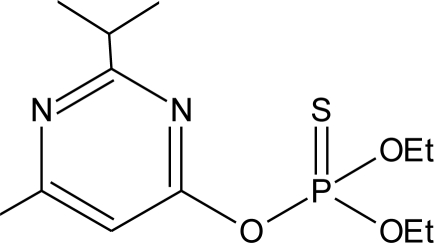 |
Estrogenic effect [115] | S: 1.84–4.96 μg/g creatinine * [69] H.S: mean 2 pg/g [85] |
|
Dichlorvos (I) M(g/Mol) = 221 pKa = n.a logP: 1.9 |
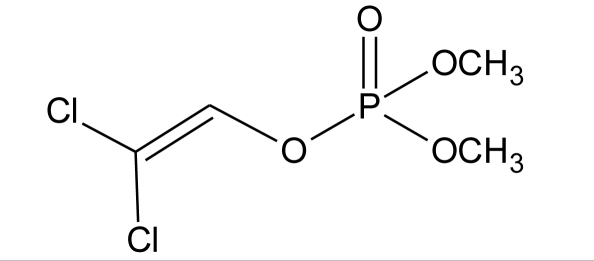 |
Weak androgen-receptor antagonist [8] | |
|
Dicofol (I) M(g/Mol) = 370.5 pKa = n.a logP: 4.3 |
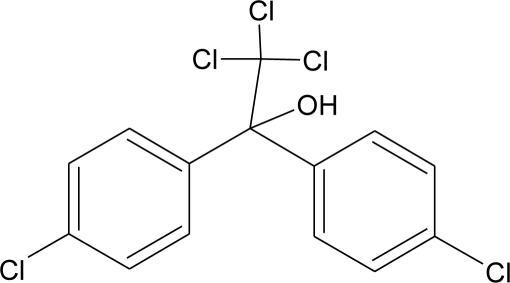 |
Inhibition of androgen synthesis, increase of estrogens synthesis, binding to estrogen receptor [90,83] | |
|
Dieldrin (I) M(g/Mol) = 380.9 pKa = n.a logP: 3.7 |
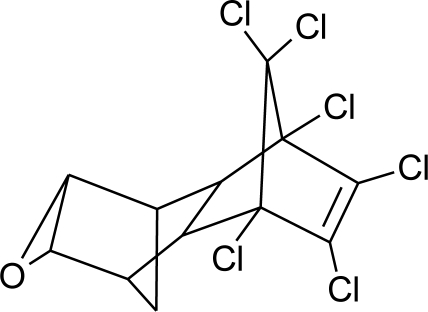 |
Competitive binding to androgen receptors, estrogenic effect, stimulation of estrogen receptor production [8,76,108,116] | H.M: <0.1–64 ng/g lipid [111] |
|
Diflubenzuron (I) M(g/Mol) = 310.7 pKa = n.a logP: 3.89 |
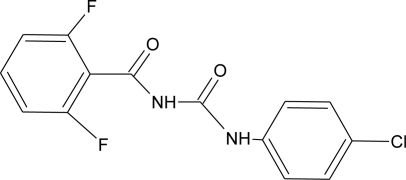 |
Pregnane X cellular receptor activation [11] | H.S: 1.21–356.4 μg/L [77,78] H.M: mean 0.66 mg/L ± 1.75 [79] A.T: 17.01–84.05 [78] |
|
Dimethoate (I) M(g/Mol) = 229.3 pKa = n.a logP: 0.704 |
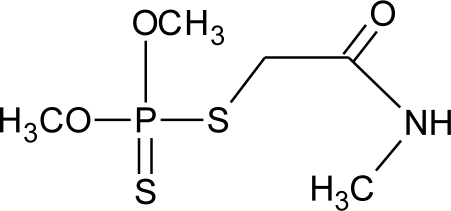 |
Disruption of thyroid hormones action. Increase of insulin blood concentration, decrease of luteinizing hormone blood concentration [117,118] | |
|
Diuron (H) M(g/Mol) = 233.1 pKa = n.a logP: 2.87 |
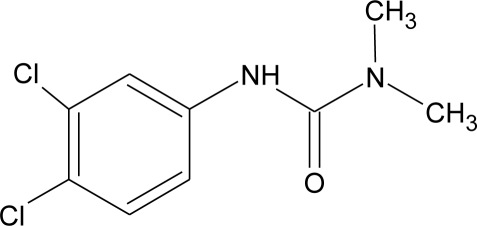 |
Inhibition of androgens action [83] | |
|
Endosulfan (I) M(g/Mol) = 406.9 pKa = n.a logP: 4.75 |
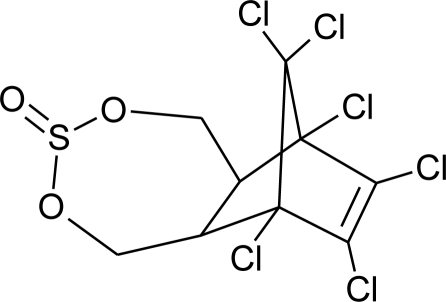 |
Competitive binding to androgen receptors, estrogenic effect, stimulation of estrogen receptor production, inhibition of aromatase activity [8,76,109,116] | H.S: 8.85 547.6 μg/L [77,78] A.T: 21.4 417.6 ng/g lipid [78] |
|
Endrin (I) M(g/Mol) = 380.9 pKa = n.a logP: 3.2 |
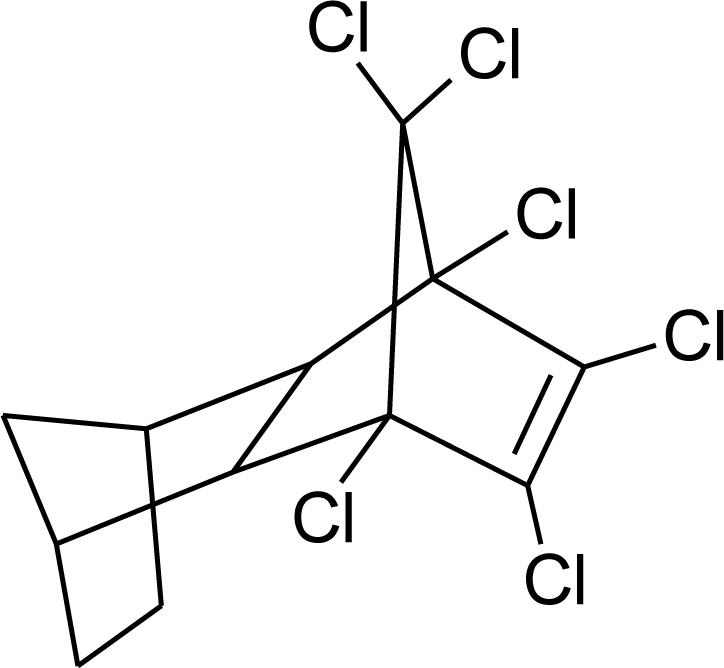 |
Competitive binding to androgen receptors [76] | H.M: mean 0.65 mg/L ± 1.63 [79] H.S: 1.21 6.35 μg/L [78] A.T: 47.43 148.13 ng/g lipid [78] |
|
Epoxyconazole (F) M(g/Mol) = 329.8 pKa = n.a logP: 3.3 |
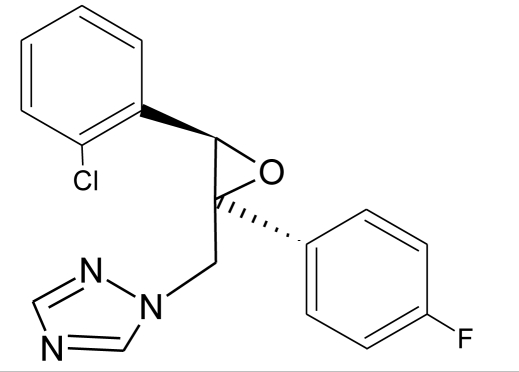 |
Inhibition of aromatase activity, decrease of estrogen production and increase available androgens [89,119] | |
|
Fenarimol (F) M(g/Mol) = 331.2 pKa = n.a logP: 3.69 |
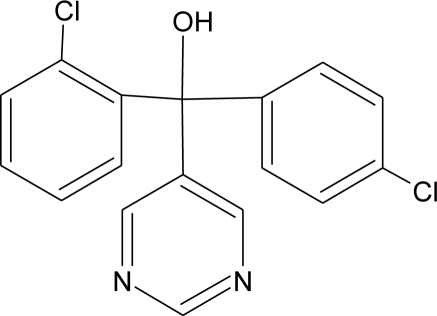 |
Antagonist of androgenic action. Potential aromatase inhibition. Pregnane X cellular receptor activation [8,11,120] | |
|
Fenbuconazole (F) M(g/Mol) = 336.8 pKa = n.a logP: 3.79 |
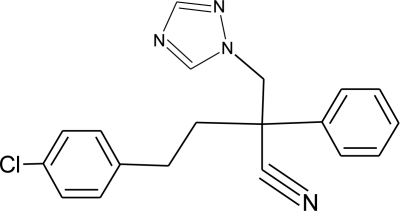 |
Inhibition of thyroid hormones production, Pregnane X cellular receptor activation [11,13] | |
|
Fenitrothion (I) M(g/Mol) = 277.2 pKa = n.a logP: 3.32 |
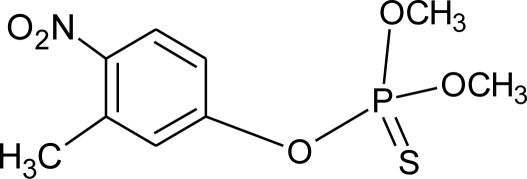 |
Competitive binding to androgen receptor, inhibition of estrogens action [90,121] | H.S: 4.5 μg/mL [71] ** |
|
Fenoxycarb (I) M(g/Mol) = 301.3 pKa = n.a logP: 4.07 |
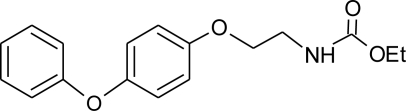 |
Interference with testosterone metabolism [122] | |
|
Fenvalerate (I) M(g/Mol) = 419.9 pKa = n.a logP: 5.01 |
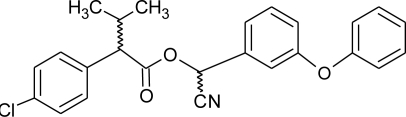 |
Inhibition of estrogen-sensitive cells proliferation, antagonist of the progesterone action [86,123] | |
|
Fluvalinate (I) M(g/Mol) = 502.9 pKa = n.a logP: 3.85 |
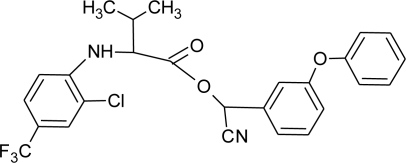 |
Binding to human sex hormone, Inhibition of progesterone production [124,125] | |
|
Flusilazole (F) M(g/Mol) = 315.4 pKa = 2.5 logP: 3.87 |
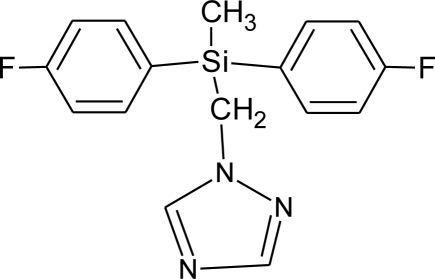 |
Inhibition of aromatase activity, decrease of estrogens production, increase of available androgens [89] | |
|
Flutriafol (F) M(g/Mol) = 301.3 pKa = 2.3 logP: 2.3 |
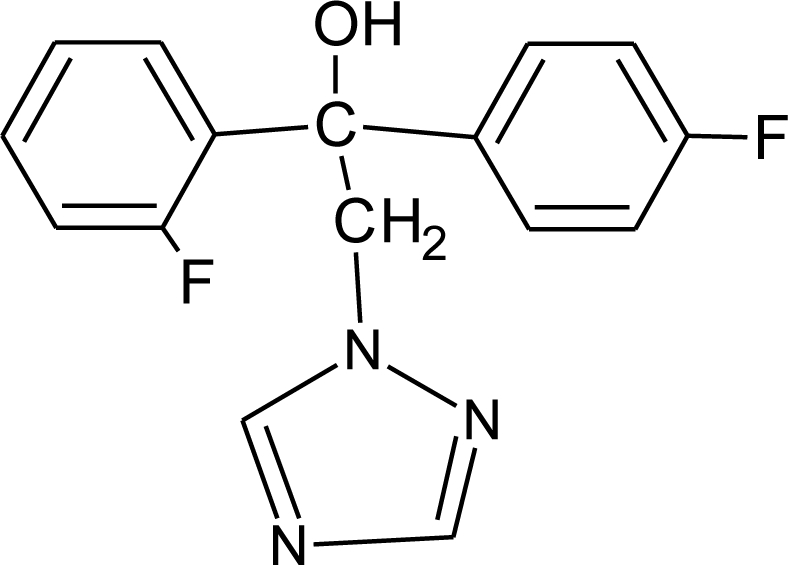 |
Weak estrogen inhibition [119] | |
|
Glyphosphate (H) M(g/Mol) = 168.1 pKa = 0.78; 2.34; 5.96; 10.98 logP: −3.2 |
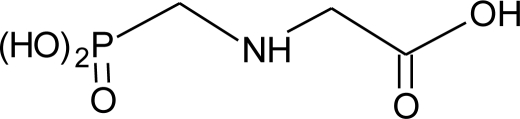 |
Disruption of aromatase activity, preventing the production of estrogens [126] | U: 1.1–2.1 ng/mL [84] |
|
HCB (F) M(g/Mol) = 284.8 pKa = n.a logP: 3.93 |
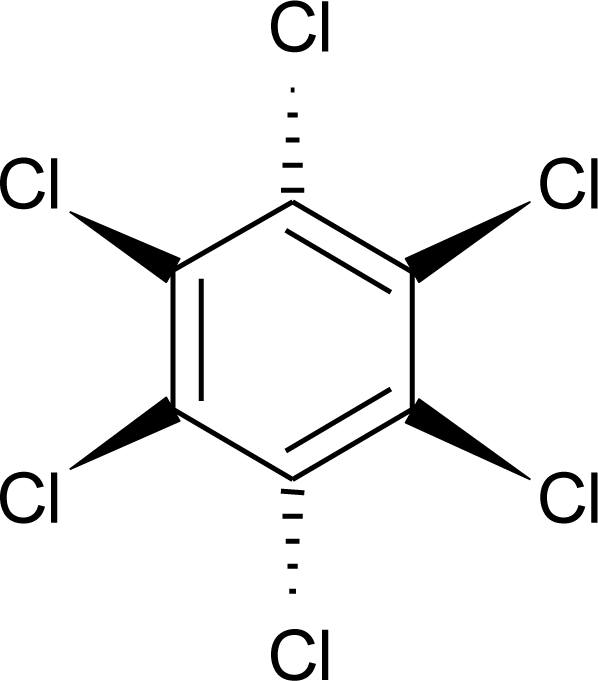 |
Severely disruption of thyroid hormone production. Enhancement of androgen action at low doses, but inhibition at high levels [127,128] | M.P: 1.6–44.3 ng/g lipid [94] B.S: 7.4–37.2 ng/g lipid [95] H.M: 0.4–472 ng/g lipid [79,96–99] H.S: 12.5–393.3 μg/L [77] H 1.2–15.9 pg/mg [113] F.F: 0.11–0.2 ng/L [100] A.F: [112] |
|
HCH (lindane) (I) M(g/Mol) = 290.8 pKa = n.a logP: 3.61 |
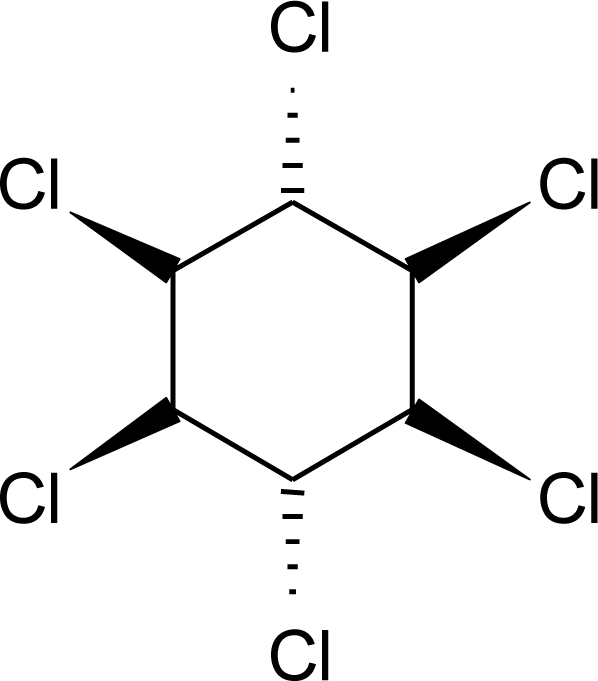 |
Reduction of oestrous cycles and luteal progesterone concentrations. Increase of insulin and estradiol blood serum concentrations, decrease thyroxine concentrations. Competitive binding to AR, ER and PR [117,129] | M.P: 0.4–2839 ng/g lipid [94] B.S: <LOD–134 ng/g lipid [95] H.M: 4.7–8700 ng/g lipid [80,110,96–99] H.S: 1.08–265.8 μg/L [77,78] H: 50.7–235 pg/mg [113] A.T: 17.44–113.31 ng/g lipid [78] M.B: 1.9–386.6 ng/g lipid [110, 114] A.F: 0.1–0.26 ng/mL [112] U.C: 4–130 ng/g lipid [110] |
|
Heptachlor (I) M(g/Mol) = 373.3 pKa = n.a logP: 5.44 |
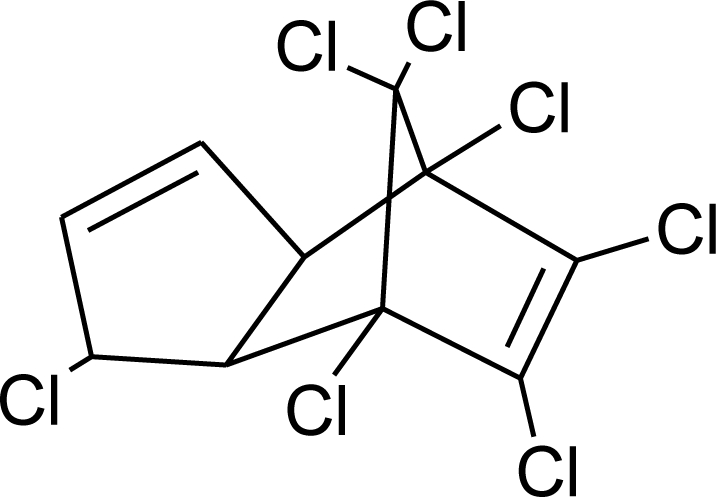 |
Binding to cellular estrogen and androgen receptors [130,131] | M.P: 0.2–5.2 ng/g lipid [94] B.S: <LOD–0.9 ng/g lipid [95] Human serum: 12.5–139.1 μg/L [77] H.M: mean 0.07 mg/L ± 0.34 [79] |
|
Hexaconazole (F) M(g/Mol) = 314.2 pKa = 2.3 logP: 3.9 |
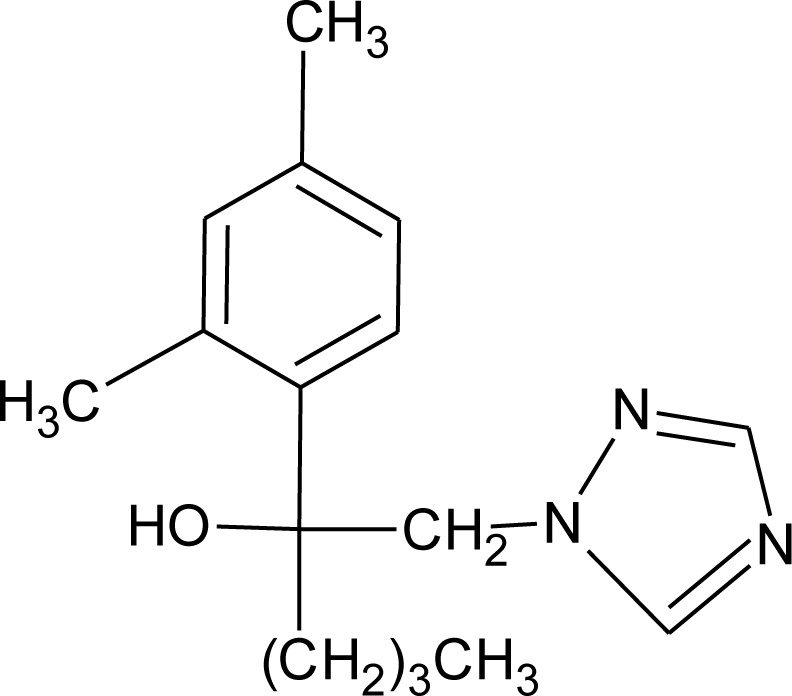 |
Inhibition of aromatase activity, decrease of the estrogens production and increase of available androgens [89] | |
|
Isoproturon (H) M(g/Mol) = 206.3 pKa = n.a logP: 2.5 |
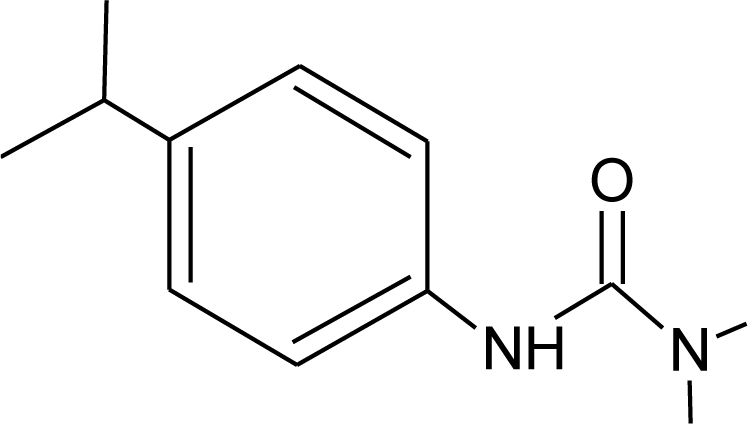 |
Pregnane X cellular receptor activation [11] | |
|
Iprodione (F) M(g/Mol) = 330.2 pKa = n.a logP: 3.1 |
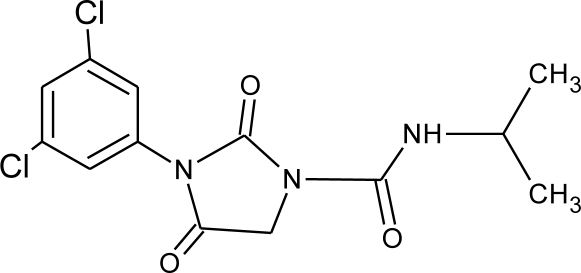 |
Increase weakly aromatase activity, and estrogen production [8] | |
|
Linuron (H) M(g/Mol) = 249.1 pKa = n.a logP: 3 |
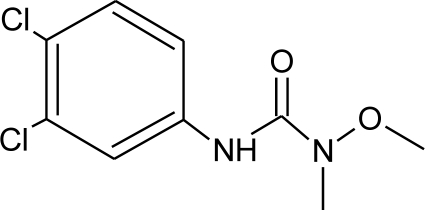 |
Competitive binding to androgen receptor, thyroid receptor agonist [131,132] | |
|
Malathion (I) M(g/Mol) = 330.4 pKa = n.a logP: 2.75 |
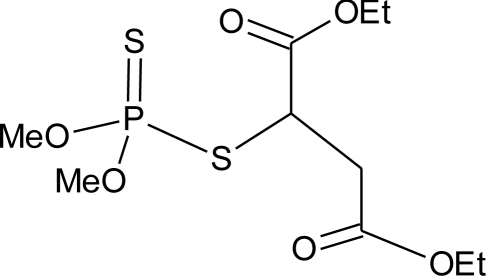 |
Inhibition of catecholamine secretion, binding to thyroid hormone receptors [13,133] | U: <LOD–3195 ng/mL [68] * M: 2.92–5.38 μg/mL [88] H: 1.62–2.12 μg/mL [88] S: 0.37–0.92 μg/g creatinine [69] |
|
Methiocarb (H) M(g/Mol) = 225.3 pKa = n.a logP: 3.18 |
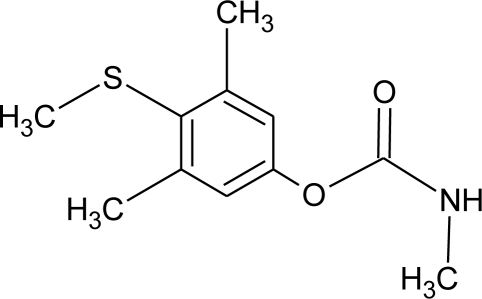 |
Inhibition of androgen activity and increase of estrogen one [8] | |
|
Methomyl (I) M(g/Mol) = 162.2 pKa = n.a logP: 1.24 |
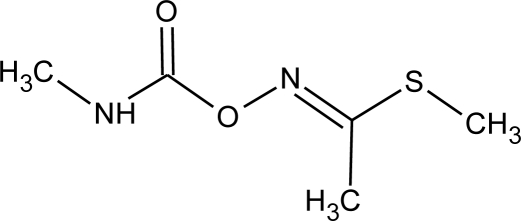 |
Weak increase of aromatase activity and estrogen production [8,13] | |
|
Methoxychlor (I) M(g/Mol) = 345.7 pKa = n.a logP: 5.83 |
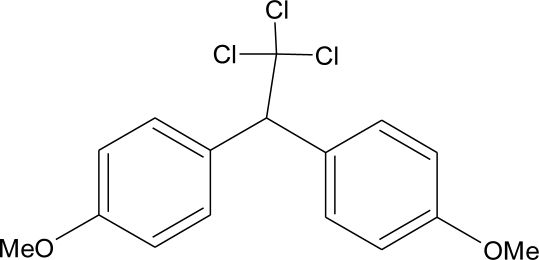 |
Strong estrogenic effect. Competitive binding to androgen receptor, interaction with the pregnane X cellular receptor [13,74,76] | H.S: 0.38–0.39 μg/L [78] A.T: 29.86–155.58 ng/g lipid [78] |
|
Metolachlor M(g/Mol) = 283.8 pKa = n.a logP: 3.4 |
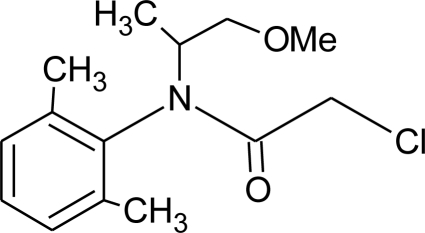 |
Pregnane X cellular receptor activation [11] | U: <LOD–4.5 ng/mL [68,84] H.S: mean 2 pg/g [85] M.S: 0.007–1.96 ng/g [92] U.C: 0.007–2.37 ng/g [92] S: 0.20–0.48 μg/g creatinine [69] |
|
Metribuzin (H) M(g/Mol) = 214.3 pKa = 0.99 logP: 1.65 |
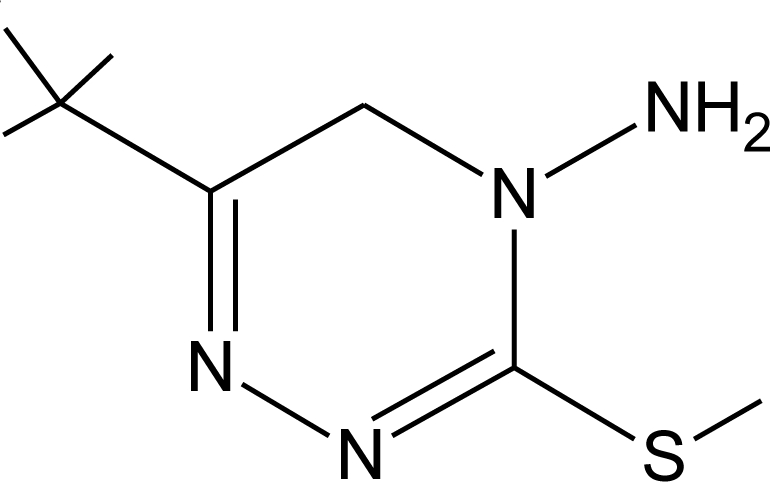 |
Hyperthyroidism, alteration of somatotropin levels [134] | |
|
Mirex (I) M(g/Mol) = 545.5 pKa = n.a logP: 5.28 |
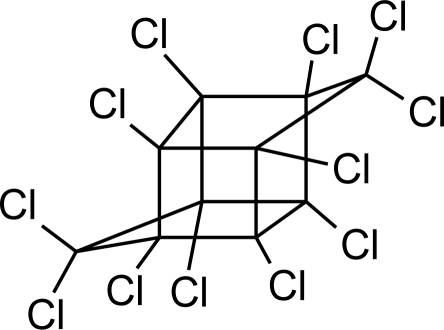 |
Weak estrogen effect [13] | M.P: 0.2–1.5 ng/g lipid [94] B.S: <LOD–7.2 ng/g lipid [95] H.M: 0.2–1.7 ng/g lipid [98] |
|
Molinate (H) M(g/Mol) = 187.3 pKa = n.a logP: 2.86 |
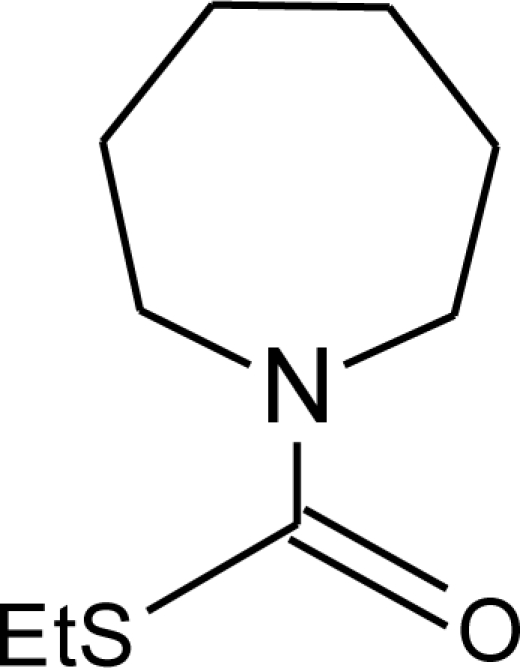 |
Reproductive tract damage, reduction of fertility [13] | |
|
Myclobutanil (F) M(g/Mol) = 288.8 pKa = 2.3 logP: 2.89 |
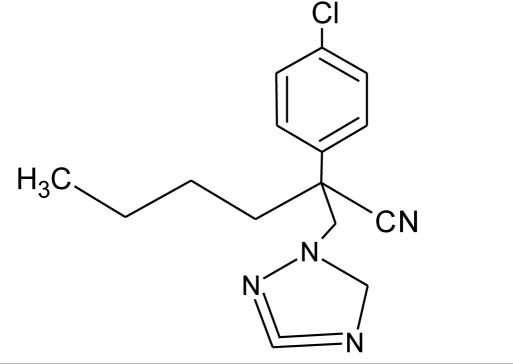 |
Weak estrogen and androgen inhibition, Binding to estrogen and androgen receptors, aromatase inhibition [89,90,119] | |
|
Nitrofen (H) M(g/Mol) = 284.1 pKa = n.a logP: 3.4 |
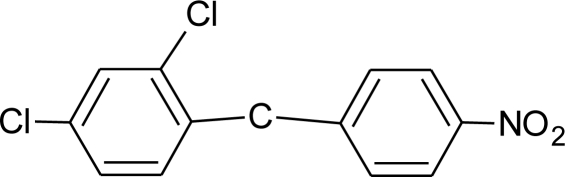 |
Estrogen and androgen inhibition [90] | |
|
Oxamyl (I) M(g/Mol) = 219.3 pKa = n.a logP: −0.44 |
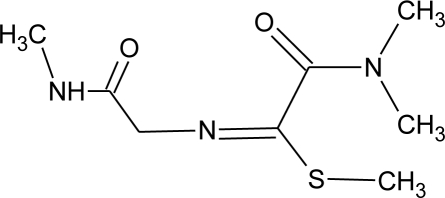 |
Weak estrogen effect [13] | |
|
Parathion (I) M(g/Mol) = 291.3 pKa = n.a logP: 3.83 |
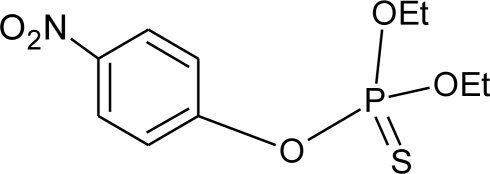 |
Inhibition of catecholamine secretion, increase of melatonin synthesis, inhibition of gonadotrophic hormone [13] | U: <LOD–84 ng/mL * [68] |
|
Penconazole (F) M(g/Mol) = 284.2 pKa = 1.51 logP: 3.72 |
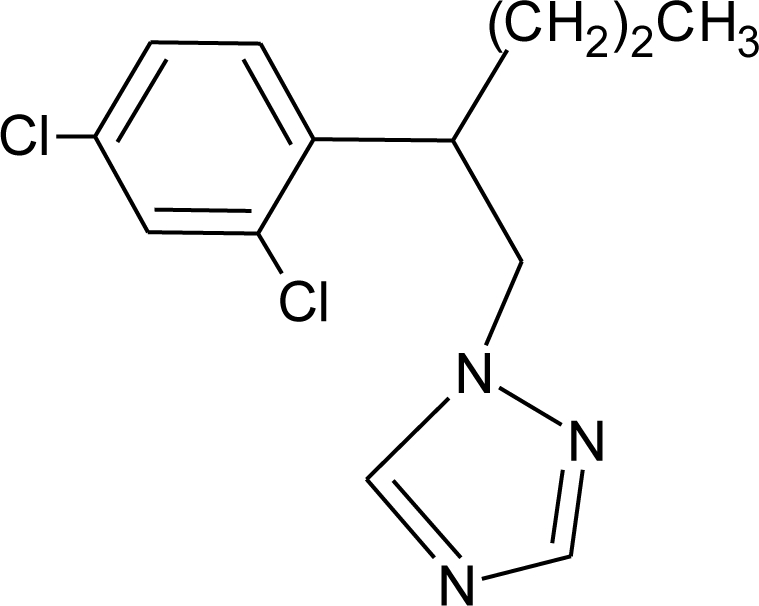 |
Weak estrogenic effect. Inhibition of aromatase activity, decrease of estrogens production and increase androgens availability [89,119] | |
|
Pentachlorophenol (H, F, I) M(g/Mol) = 266.3 pKa = 4.73 logP: 3.32 |
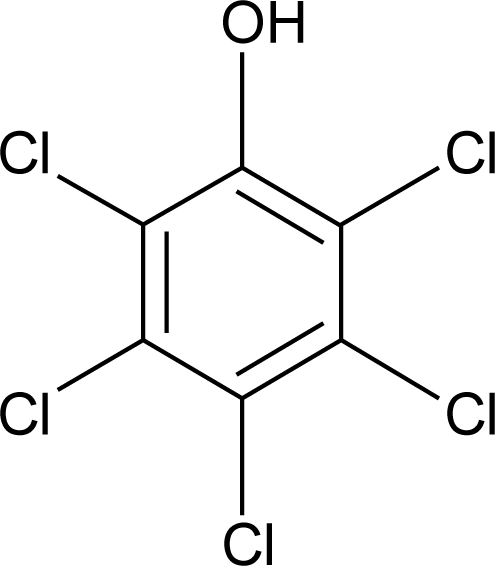 |
Weak estrogenic and anti-androgenic affect [13] | A.F : 0.15–0.54 ng/mL [135] |
|
Permethrin (I) M(g/Mol) = 391.3 pKa = n.a logP: 6.1 |
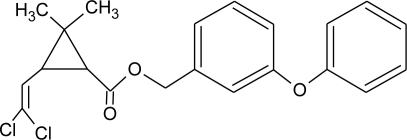 |
Inhibition of estrogen-sensitive cells proliferation [87,106] | U: 1–150 μg/g * [107] |
| Phenylphenol (F) M(g/Mol) = 170.2 pKa = 9.97 logP:3.09 |
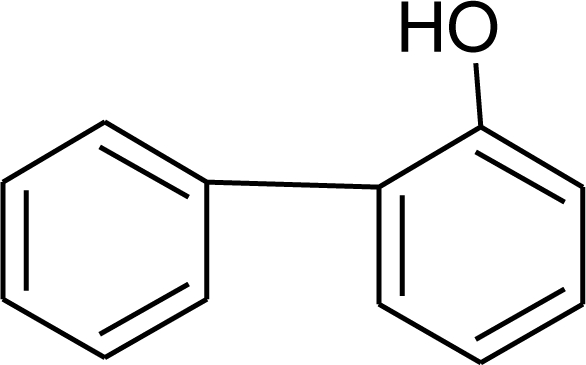 |
Estrogen agonist [136] | A.F: 0.1–0.17 ng/mL [135] |
|
Prochloraz (F) M(g/Mol) = 376.7 pKa = 3.8 logP: 3.53 |
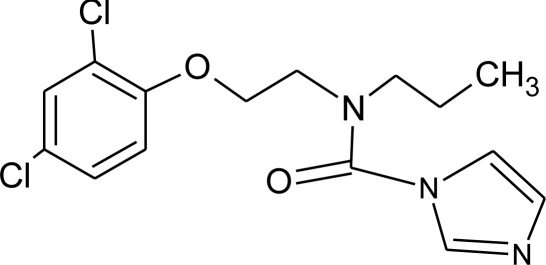 |
Activation of Pregnane X cellular receptor. Antagonist to cellular androgen and estrogen receptors, agonist to Ah receptor and inhibition of aromatase activity [8,11,120,137] |
|
|
Procymidone (F) M(g/Mol) = 284.1 pKa = n.a logP: 3.3 |
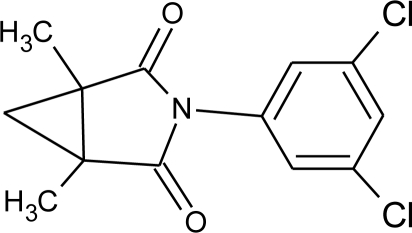 |
Competitive binding to androgen receptor [131] | |
|
Propamocarb (F) M(g/Mol) = 188.3 pKa = 9.5 logP: 0.84 |
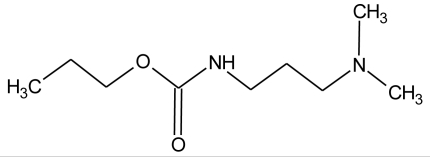 |
Weak increase of aromatase activity and estrogen production [8] | |
|
Propanil (H) M(g/Mol) = 318.1 pKa = n.a logP: 2.29 |
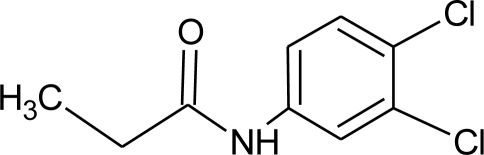 |
Increase of cellular response to estrogen [138] | |
|
Propazine (H) M(g/Mol) = 229.8 pKa = 1.7 logP: 3.95 |
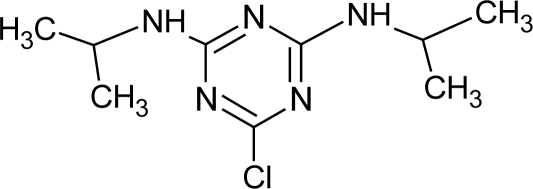 |
Induction of aromatase activity and increase of estrogen production [81] | |
|
Propiconazole (F) M(g/Mol) = 342.2 pKa = 1.09 logP: 3.72 |
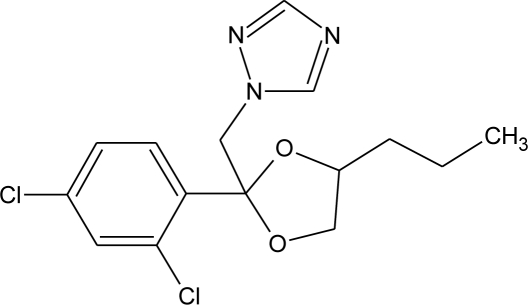 |
Weak estrogen and aromatase activity inhibition. Decrease estrogens production and increase of androgens availability [89,119] |
|
|
Propoxur (I) M(g/Mol) = 209.2 pKa = n.a logP: 0.14 |
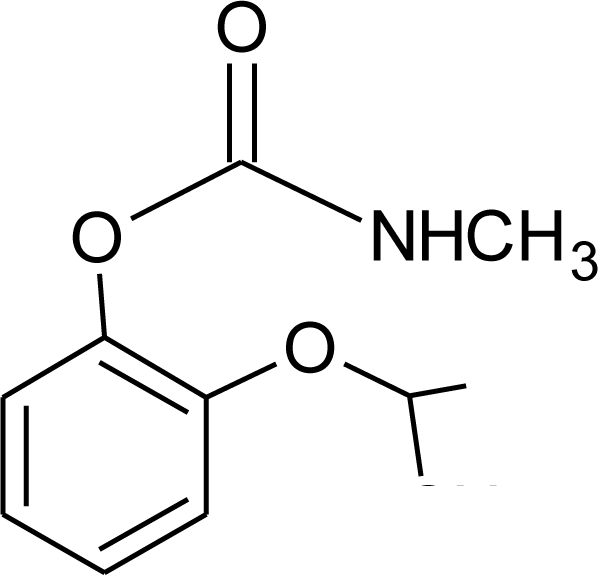 |
Weak estrogenic effect [13] | M: 0.24–1.50 μg/mL [88] C.B: 0.77 μg/mL [88] H: 0.22–0.42 μg/mL [88] M.B: 0.67–0.77 μg/mL [88] |
|
Prothiophos (I) M(g/Mol) = 345.3 pKa = n.a logP: 5.67 |
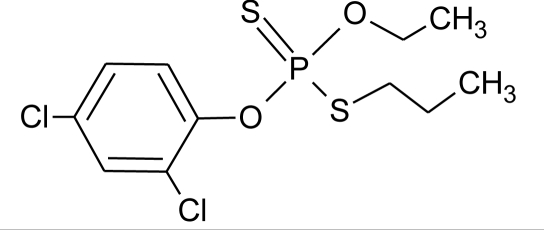 |
Estrogenic effect [115] | |
|
Pyridate (H) M(g/Mol) = 378.9 pKa = n.a logP: 0.5 |
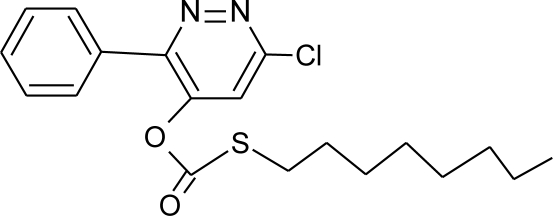 |
Binding to estrogen and androgen receptors [90] | |
|
Pyrifenox (F) M(g/Mol) = 295.2 pKa = 4.61 logP: 3.4 |
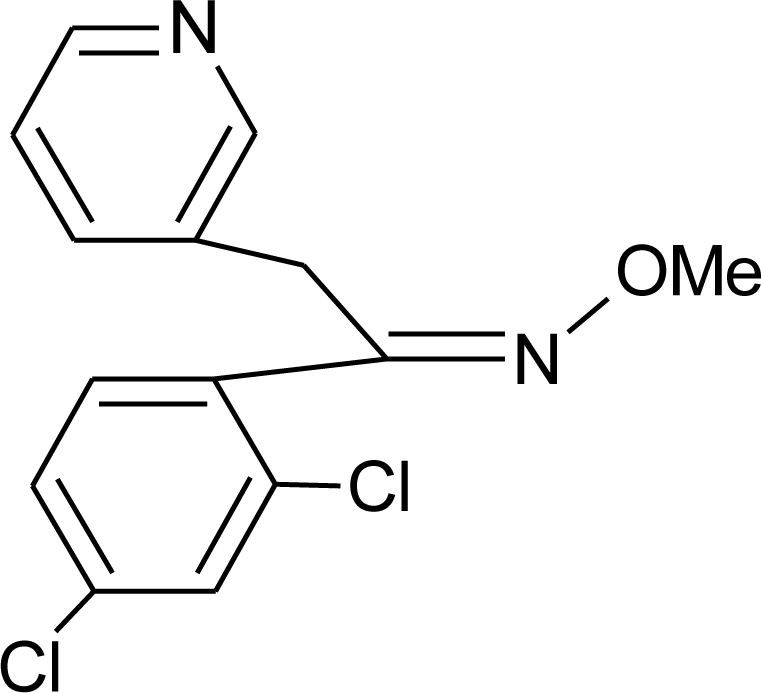 |
Weak estrogen inhibition [119] | |
|
Pyripyroxifen (I) M(g/Mol) = 321.4 pKa = 6.87 logP: 5.37 |
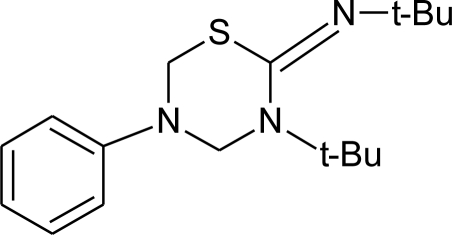 |
Estrogenic effect [115] | |
|
Resmethrin (I) M(g/Mol) = 338.4 pKa = n.a logP: 5.43 |
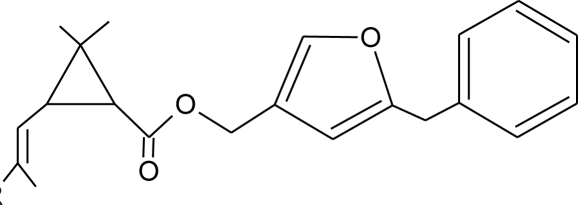 |
Binding to sex hormone [124] | |
|
Simazine (H) M(g/Mol) = 201.7 pKa = 1.62 logP: 2.3 |
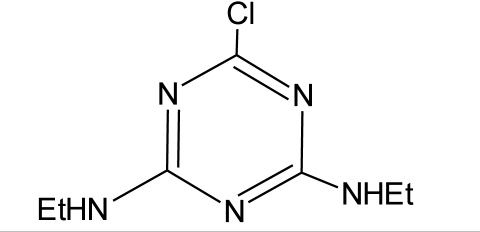 |
Induction of aromatase activity, increase of estrogen production [81] | |
|
Sumithrin (I) M(g/Mol) = 350.5 pKa = n.a logP: 6.01 |
 |
Increase of estrogen-sensitive cells proliferation, antagonist of the progesterone action [87,123] | |
|
Tebuconazole (F) M(g/Mol) = 307.8 pKa = n.a logP: 3.7 |
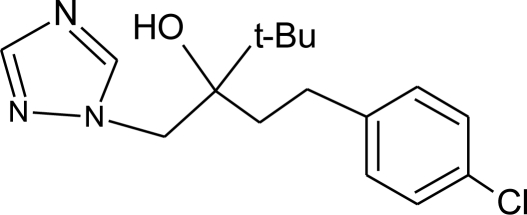 |
Inhibition of aromatase activity, decrease the estrogens production and increase androgens availability [89] | |
|
Tetramethrin (I) M(g/Mol) = 331.4 pKa = n.a logP: 4.6 |
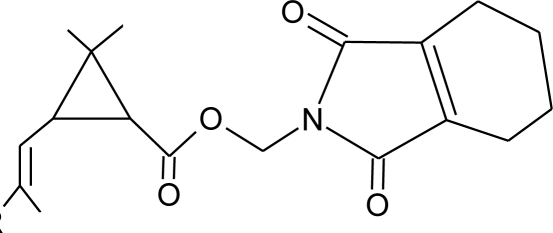 |
Estrogen-antagonistic effects in females only [139] | |
|
Tolchlofos-methyl (I) M(g/Mol) = 301.1 pKa = n.a logP: 4.56 |
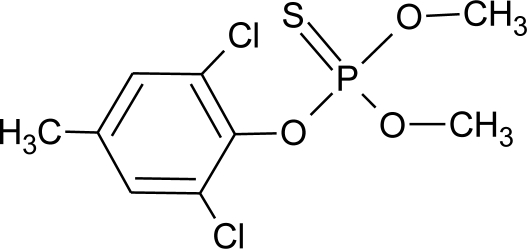 |
Competitive binding to cellular estrogen receptors [120] | |
|
Toxaphene (I) M(g/Mol) = 411.8 pKa = n.a logP: 3.3 |
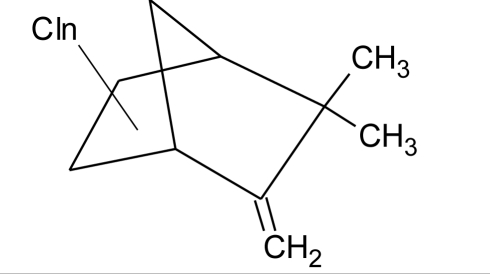 |
Increase of estrogen-sensitive cells proliferation. Inhibition of corticosterone synthesis in the adrenal cortex [13,116] |
|
|
Triadimefon (F) M(g/Mol) = 293.8 pKa = n.a logP: 3.18 |
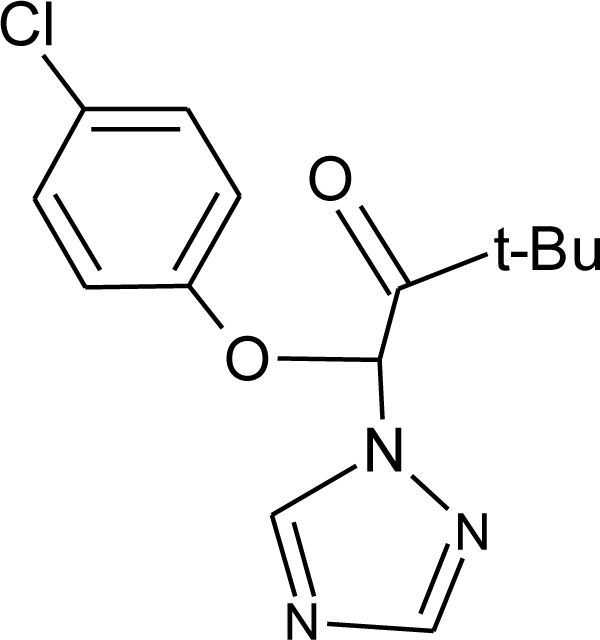 |
Estrogenic effect, inhibition of aromatase activity, decrease of estrogens production and increase androgens availability [90] | |
|
Triadimenol (F) M(g/Mol) = 295.8 pKa = n.a logP: 3.18 |
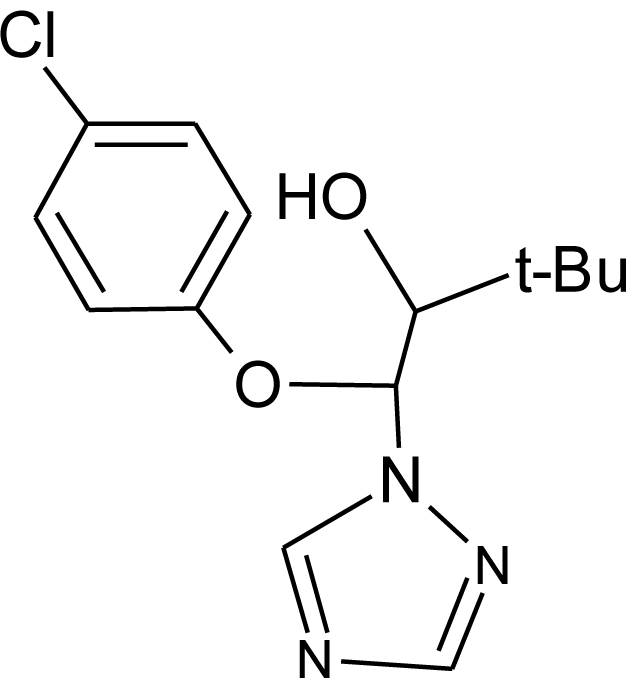 |
Estrogenic effect, inhibition of aromatase activity, decrease of estrogens production and increase androgens availability [89,90] | |
|
Tribenuron-methyl (H) M(g/Mol) = 395.4 pKa = 4.7 logP: 0.78 |
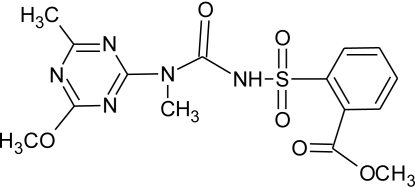 |
Weak estrogenic effect [8] | |
|
Trichlorfon (I) M(g/Mol) = 257.4 pKa = n.a logP: 0.43 |
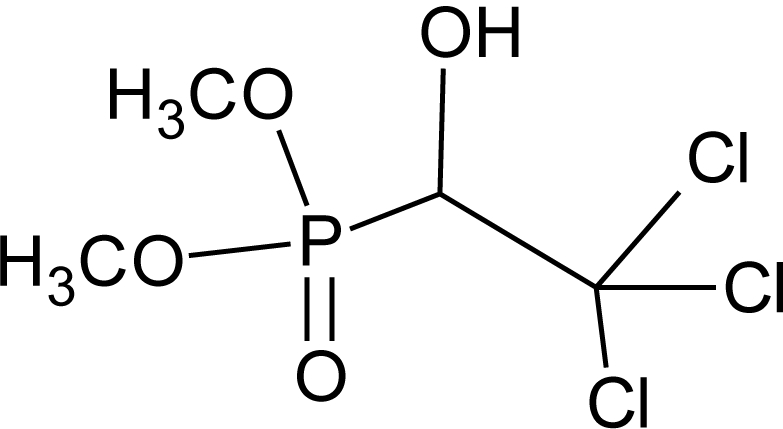 |
Alteration of thyroid function [140] | |
|
Trifluralin (H) M(g/Mol) = 335.3 pKa = n.a logP: 5.27 |
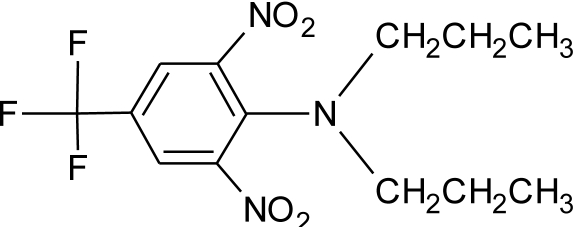 |
Interaction withs pregnane X cellular receptor, interference steroid hormone metabolism [74] | M.S: 0.00–8.5 ng/g [92] U.C: 0.007–4.42 ng/g [92] |
|
Vinclozolin (F) M(g/Mol) = 286.1 pKa = n.a logP: 3.02 |
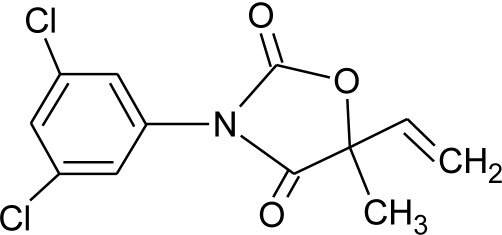 |
Competitive binding to androgen receptor Interactions with pregnane X cellular receptor, interference with steroid hormone metabolism. [8,74,131] |
(H): Herbicide, (F): Fungicide, (I): Insecticide, (AFA): Antifouling agent, (T): Termiticide, U: urine, S: semen, H.S: human serum, H.M human milk, M: mecomium, H = hair, A.T: adipose tissues, F.F: follicular fluid, M.P: maternal plasma, U.C: umbilical cord.
Measured by the presence of its metabolite.
Case of poisoning patient.
