Abstract
Study Objectives:
To test the hypotheses that the dynamic changes in brain oxygen partial pressure (PtO2) in response to obstructive apneas or to intermittent hypoxia differ from those in other organs and that the changes in brain PtO2 in response to obstructive apneas is a source of oxidative stress.
Design:
Prospective controlled animal study.
Setting:
University laboratory.
Participants:
98 Sprague-Dawley rats.
Interventions:
Cerebral cortex, skeletal muscle, or visceral fat tissues were exposed in anesthetized animals subjected to either obstructive apneas or intermittent hypoxia (apneic and hypoxic events of 15 s each and 60 events/h) for 1 h.
Measurements and Results:
Arterial oxygen saturation (SpO2) presented a stable pattern, with similar desaturations during both stimuli. The PtO2 was measured by a microelectrode. During obstructive apneas, a fast increase in cerebral PtO2 was observed (38.2 ± 3.4 vs. 54.8 ± 5.9 mm Hg) but not in the rest of tissues. This particular cerebral response was not found during intermittent hypoxia. The cerebral content of reduced glutathione was decreased after obstructive apneas (46.2% ± 15.2%) compared to controls (100.0% ± 14.7%), but not after intermittent hypoxia. This antioxidant consumption after obstructive apneas was accompanied by increased cerebral lipid peroxidation under this condition. No changes were observed for these markers in the other tissues.
Conclusions:
These results suggest that cerebral cortex could be protected in some way from hypoxic periods caused by obstructive apneas. The increased cerebral PtO2 during obstructive apneas may, however, cause harmful effects (oxidative stress). The obstructive apnea model appears to be more adequate than the intermittent hypoxia model for studying brain changes associated with OSA.
Citation:
Almendros I; Farre R; Planas AM; Torres M; Bonsignore MR; Navajas D; Montserrat JM. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. SLEEP 2011;34(8):1127-1133.
Keywords: Tissue oxygenation, obstructive apnea, intermittent hypoxia, animal model, oxidative stress
INTRODUCTION
Obstructive sleep apnea (OSA), which is reported in about 4% and in 2% of middle-aged men and women, respectively,1,2 is characterized by recurrent episodes of partial or complete obstruction of the upper airway during sleep, with consequent decreases in oxygen saturation. Cyclical changes in oxygen saturation mimicking OSA have been related to oxygen-reactive species production3,4 and neuronal apoptosis5–8 and/or necrosis.9 These deleterious effects caused in part by intermittent hypoxia in brain tissue can contribute to the development of cognitive impairment associated with OSA.7,10
In a rat model of obstructive apneas we recently documented a rapid increase in oxygen partial pressure (PtO2) in cerebral cortex in response to obstructive apneas, despite recurrent cyclical changes in arterial oxygen saturation (SpO2).11 The interpretation of this result was that the brain could be partially protected from recurrent obstructive apneas, probably through compensatory mechanisms such as hypercapnia, but it is not known whether this particular response to recurrent obstructive apneas in brain PtO2 also occurs in other tissues. Moreover, there are no data available to indicate whether the brain PtO2 response is similar when the animal is subjected to obstructive apneas or to intermittent hypoxia (which excludes recurrent hypercapnia).
The present study was designed to test the following hypotheses. First, the dynamic changes in brain PtO2 in response to obstructive apneas or to intermittent hypoxia differ from those in other organs on account of specific mechanisms regulating cerebral circulation.12 Second, the increased mean PtO2, with the persistence of PtO2 swings observed in brain tissue in response to obstructive apneas, is a source of oxidative stress that could explain the neurological/cognitive consequences of OSA. To this end, we measured PtO2 in cerebral cortex, skeletal muscle and visceral fat tissues in rats subjected to noninvasive recurrent obstructive apneas and to intermittent hypoxia, in both cases with the same degree of arterial oxygen desaturations. To assess the potential oxidative stress induced by obstructive apneas and by intermittent hypoxia, consumption of endogenous antioxidant reduced glutathione (GSH) and lipid peroxidation in tissues was also measured.
MATERIALS AND METHODS
Animal Preparation
This study was carried out on 98 Sprague-Dawley male rats (300-350 g) and was approved by the Animal Experimentation Ethical Committee (CEEA) of the University of Barcelona. Fifty rats were anesthetized intraperitoneally with urethane 10% (1 g/kg), and the skeletal muscle (gracilis muscle) (n = 17), visceral (intra-abdominal) fat (n = 17), or cerebral cortex (2 mm anterior to bregma and 2 mm left from midline) (n = 16), tissues were exposed for PtO2 measurement. Arterial blood saturation (SpO2) was measured by a pulse oximeter positioned in the rat leg (504; Critical Care Systems, Inc., Waukesha, WI), and the rats were randomly subjected to either obstructive apneas (n = 24) or intermittent hypoxia (n = 26). In another 2 groups, each with 24 animals, markers of oxidative stress and hypoxic activation were assessed in different tissues.
Tissue Oxygen Partial Pressure Measurements
Real-time PtO2 measurements were made in the tissues of the anesthetized animals by a modified Clark's polarographic fast-response oxygen micro-electrode pipette (OX-50, Unisense A/S, Denmark; 50 μm tip diameter, 90% response time < 2 s). The spatial resolution is equal to the outside tip diameter of the sensor. The oxygen micro-electrode was connected to an amplified picoammeter (Unisense A/S, Denmark) and then was calibrated in water with 100% O2, 21% O2, and oxygen-free solutions (NaOH 0.1 M, sodium ascorbate 0.1 M; MicOX software, Unisense A/S, Denmark). The oxygen sensor was inserted 2 mm from the tissue surface by a micrometric positioner and then slightly retracted (≈ 0.5 mm). The basal value of PtO2 had to be within the 30-50 mm Hg range and also remained stable before experiments. PtO2 and SpO2 signals were continuously sampled at 60 Hz and stored for subsequent analysis.
Application of Obstructive Apneas and Intermittent Hypoxia
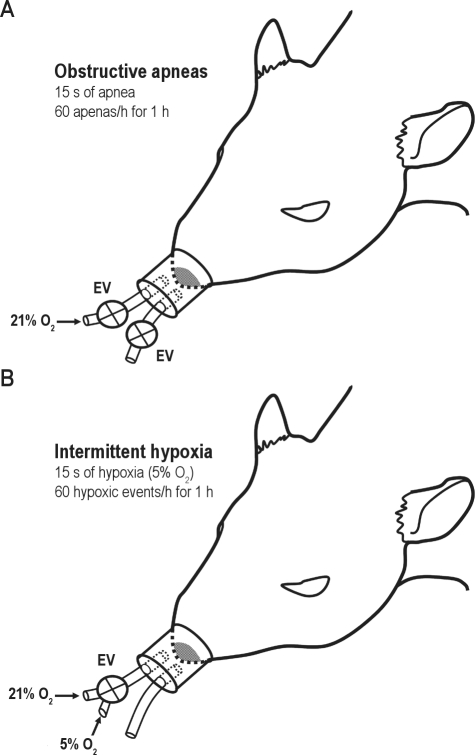
Twenty-four animals were subjected to recurrent obstructive apneas with a duration of 15 s each and 60 apneas/h for 1 h during PtO2 measurements of cerebral cortex (n = 8), skeletal muscle (n = 8), and visceral fat (n = 8) tissues. The obstructive apnea model used was based on a previously described method13 which mimicked the cyclical airway obstructions and oxygen desaturations observed in severe OSA patients.14 Briefly, this model consists of a nasal mask with 2 tubes, one open to the atmosphere and the other connected to an airflow source to avoid rebreathing during spontaneous breathing. Electrovalves positioned in each tube were synchronically closed to generate controlled obstructive apneas (Figure 1A).
Figure 1.
Experimental setup. Anesthetized animals were subjected to recurrent obstructive apneas (A) or to intermittent hypoxia test (B). EV, electrovalves. See text for explanation.
In another group of 26 rats, the animals were subjected to intermittent hypoxia for 1 h during PtO2 measurements of cerebral cortex (n = 8), skeletal muscle (n = 9), and visceral fat (n = 9) tissues. To this end, the noninvasive model for applying obstructive apneas was modified to apply intermittent hypoxia. In this case, one tube of the nasal mask remained open to the atmosphere, but the other tube was connected to a controlled 3-way electrovalve to connect the input of the nasal mask with either room air (21% O2) or hypoxic air with 5% O2 (Figure 1B). The timing used for intermittent hypoxia was identical to that used during obstructive apneas, reaching similar values of SpO2 desaturations (Figure 2A).
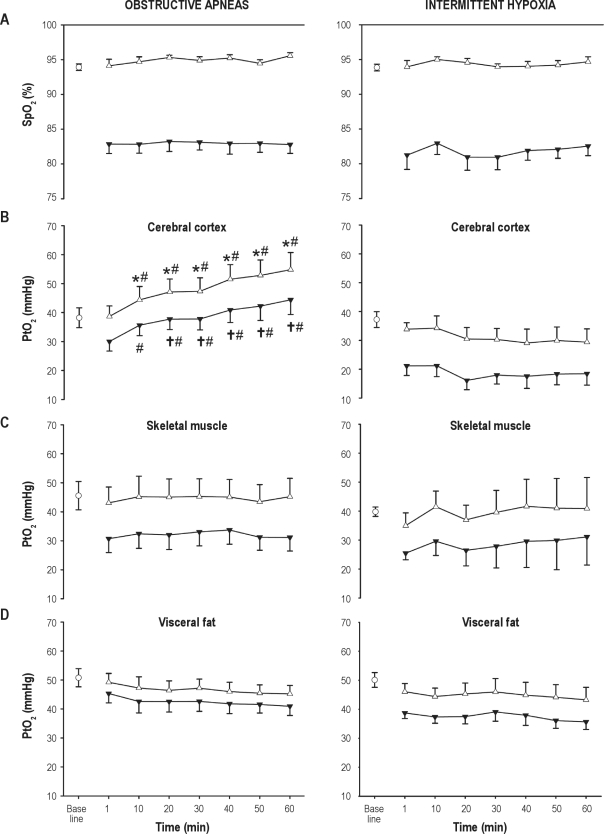
Figure 2.
Time course of the maximum (Δ) and minimum (▾) values of arterial oxygen saturation (SpO2) (A) and oxygen partial pressure (PtO2) in cerebral cortex (B), skeletal muscle (C), and visceral fat (D) during application of recurrent obstructive apneas or intermittent hypoxia. Baseline value is represented by an open circle. Results are shown as mean ± SE. Time dependence within obstructive apneas or intermittent hypoxia groups: *P < 0.05 respect with to baseline, †P < 0.05 respect with to the first apneic event. Comparisons between obstructive apneas vs. intermittent hypoxia for corresponding values in each tissue: #P < 0.05.
Measurement of Glutathione, Vascular Endothelial Growth Factor, and Hsp-70 in Tissues
A second series of 24 animals was anesthetized and subjected to obstructive apneas (n = 8), intermittent hypoxia (n = 8), or control (spontaneously breathing room air; n = 8), according to the protocol described above but without exposing the tissues or inserting the PtO2 microelectrode, thus avoiding any surgical intervention. After 1 h, the animals were sacrificed by exsanguination, and tissue samples were excised and stored at −80°C for further measurement of the levels of GSH, vascular endothelial growth factor (VEGF), and heat shock protein (Hsp-70).
The cortex of one brain hemisphere was dissected out and a portion of visceral fat and gracilis muscle were also obtained for biochemical determinations. Total (GSx) and oxidized (GSSG) glutathione concentration (nmol/mg of protein) were measured as described,15 with minor modifications.16 Tissue was sonicated in 3.3% 5-sulfosalicylic acid (1 mL per 200 mg of tissue) and centrifuged at 12,000 xgfor 30 min at 4°C. The supernatant was used to determine GSx and GSSG, whereas the pellet was used to determine total protein concentration (Bradford Method, Bio-Rad). The reaction is based on the oxidation of DTNB (5,5'-dithio-bis-(2-nitrobenzoic acid)) followed by measurement at 405-412 nm in a spectrophotometer. The formation of GSSG was determined in 100 μL of the supernatant after the addition of 2 μL of 2-vinylpyridine. The content of GSH was estimated as follows: GSH = GSx − (2*GSSG). Determination of GSH was also done from visceral fat and skeletal muscle samples.
The cortex of the remaining hemisphere was used for protein extraction and Western blotting. Total protein was extracted using radioimmunoassay buffer (RIPA) containing 0.01M PBS, sodium dodecyl sulphate, sodium deoxycholate, the non-ionic detergent Igepal, and the complete protease inhibitor cocktail (Boehringer Mannheim, Mannheim, Germany). The protein concentration of samples (solubilized in 800 μL of NaOH 0.2 N) was determined with the Bradford assay (Bio-Rad, Hercules CA, USA). Twenty-five μg of the protein extracts were run in denaturing 10% polyacrylamide gels and were transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA, USA). Membranes were incubated overnight at 4°C with one of the following primary antibodies: Anti-Hsp72/73 (Ab-1) mouse mAb (Hsp-70) (Calbiochem) diluted 1:2000, and a rabbit polyclonal antibody against VEGF (#ab46154, Abcam) diluted 1:500. A mouse monoclonal antibody against β-actin (Clone AC-15, Sigma-Aldrich) diluted 1:100,000 was used as loading control. The optical density of the bands was measured by densitometric analysis (Versadoc, Bio-Rad). Band intensity was divided by the intensity of β-actin to correct for differences in the amount of protein loaded in each gel. Samples were loaded into several gels that always contained samples from the 3 experimental groups. Then, to correct for differences in the exposure of different blots, the percent changes versus controls within each blot were calculated. In this way, samples were normalized allowing for comparisons using several blots.
Lipid Peroxidation Measurements
In a third series of 24 animals, treatment and groups identical to the second series, brain and skeletal tissue samples were used for measurement of lipid peroxidation as a marker of oxidative stress. Tissue lipid peroxidation was assessed by measuring the content of lipid hydroperoxide (LPO) using an Assay Kit (#705002, Cayman Chemical, USA) that measures the hydroperoxides directly utilizing the redox reactions with ferrous ions after lipid hydroperoxide extraction into chloroform. The assay was carried out following the instructions of the manufacturer.
Data Processing and Statistical Analysis
Both arterial SpO2 and PtO2 of cerebral cortex, skeletal muscle, and visceral fat tissues were measured at baseline and at the minimum and maximum attained during the first swing in PtO2 caused by obstructive apneas or intermittent hypoxia, and during the following PtO2 swings every 10 min up to 1 h. Two-way repeated measures ANOVA was used to examine the time dependence of the maximum (7 degrees of freedom [df]; baseline, first event and every 10 min up to 1 h) and minimum values (6 df; first event and every 10 min up to 1 h) of PtO2 in all the tissues investigated, and also to know whether there was a significant difference between intervention (obstructive apneas vs. intermittent hypoxia) for the same time-points. Similarly to PtO2, comparisons between corresponding maximum and minimum values of SpO2 during both interventions were tested by 2-way repeated-measures ANOVA. The differences in GSH, VEGF, Hsp-70, and LPO were assessed by 1-way ANOVA analysis followed by the Dunnett multiple comparison test. All data are presented as mean ± SE.
RESULTS
SpO2 presented a stable cyclical pattern, with constant maximum and minimum values, during the entire duration of the experiment. Remarkably, no significant differences in the cyclical SpO2 desaturations were observed after the application of either obstructive apneas (from 93.9 ± 0.5 %O2 to 82.9 ± 1.3 %O2) or intermittent hypoxia stimuli (from 93.8 ± 0.5 %O2 to 81.3 ± 2.1 %O2) (Figure 2A).
When comparing intermittent hypoxia and obstructive apneas, a significantly different pattern was observed in cerebral cortex PtO2, which experienced a fast increase during the obstructive apnea protocol (Figure 2B). Moreover, the maximum PtO2 in cerebral cortex showed a significant increase from 38.2 ± 3.4 mm Hg at baseline to 54.8 ± 5.9 mm Hg after 60 min of recurrent obstructive apneas (P < 0.001, df = 7, F = 1.361). The minimum values attained at the end of the hypoxic period were also increased (P < 0.001, df = 6, F = 1.799) from 30.3 ± 3.3 mm Hg at the first apneic event to 44.4 ± 5.1 mm Hg at 60 min. Interestingly, this type of increase did not occur in those animals subjected to intermittent hypoxia, where both maximum and minimum values presented a tendency to decrease, showing (in both cases) statistical differences in the interaction between intervention (apneas/intermittent hypoxia) and time (P < 0.001, df = 7, F = 7.423 and P = 0.001, df = 6, F = 4.165, for maximum and minimum values, respectively) (Figure 2B). In contrast, skeletal muscle and visceral fat PtO2 presented a stable pattern, similar to that of SpO2, without any differences between the application of obstructive apneas or intermittent hypoxia during the 1 h studied (Figure 2C and Figure 2D).
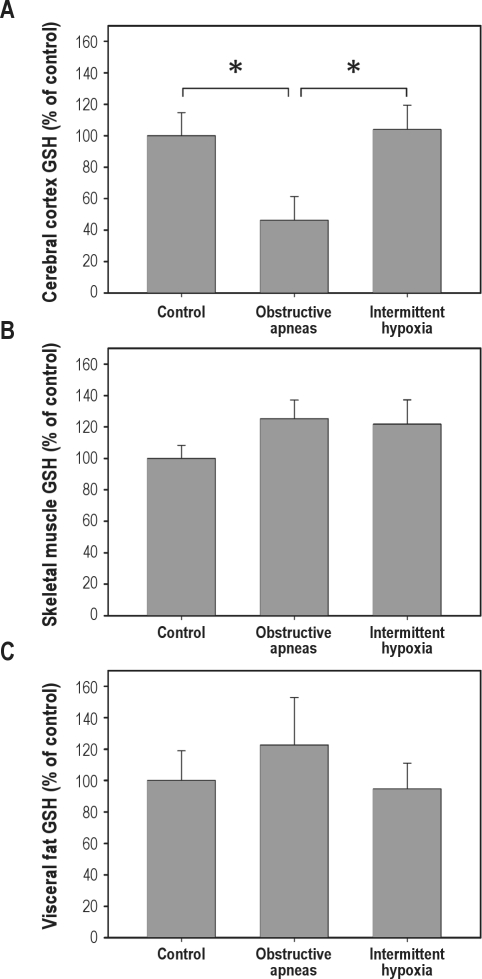
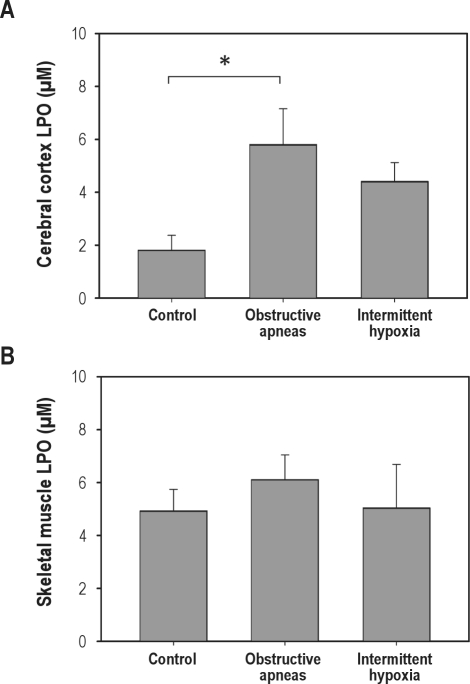
GSH in brain tissue from rats subjected to obstructive apneas (46.21% ± 15.21%) was significantly (P = 0.023) lower than in the controls (100% ± 14.67%), indicating a fast consumption of this natural antioxidant during the period of obstructive apneas. In contrast, in brain GSH levels after intermittent hypoxia (104.0% ± 15.5%) were not significantly different from controls (Figure 3A). Furthermore, the GSH content in fat (Figure 3B) and muscle (Figure 3C) did not significantly change after either obstructive apneas or intermittent hypoxia. The fast consumption of GSH in brain tissue might be due to the oxidative stress generated by the increased brain oxygenation during obstructive apneas. In support of this, the levels of LPO were significantly increased (P < 0.05) in the brain after obstructive apneas (5.81 ± 1.35 μM) compared to control (2.07 ± 0.57 μM), whereas only a nonsignificant tendency to higher LPO levels was observed after the intermittent hypoxia challenge (4.41 ± 0.71 μM) (Figure 4A). Moreover, the increase of LPO resulting from obstructive apneas was not found in skeletal muscle samples (Figure 4B).
Figure 3.
Glutathione (GSH) levels in cerebral cortex (A), skeletal muscle (B), and visceral fat (C) tissues in control animals and in animals subjected to recurrent obstructive apneas or intermittent hypoxia. Results are shown as mean ± SE. *P < 0.05.
Figure 4.
Lipid peroxidation (LPO) levels in cerebral cortex (A) and skeletal muscle (B) tissues for all groups (control, obstructive apneas, and intermittent hypoxia). Results are shown as mean ± SE. *P < 0.05.
VEGF protein levels in brain tissue did not differ between all groups (values are expressed as percentage of controls and are the group mean ± SE): obstructive apneas (88.9% ± 6.5%), intermittent hypoxia (87.7% ± 3.6%), and control animals (100% ± 5.1%). Likewise, no changes were found in the expression of Hsp-70 between these conditions: obstructive apneas (117.3% ± 25.1%), intermittent hypoxia (72.8% ± 11.6%), and control groups (100% ± 15.9%).
DISCUSSION
This study reveals a rapid recovery of PtO2 values in cerebral cortex, but not in the other tissues analyzed in animals subjected to recurrent obstructive apneas. In contrast, this particular brain tissue response was not found in animals subjected to intermittent hypoxia, although these data may suggest that cerebral cortex could be protected in some way from hypoxic periods caused by obstructive apneas. The decrease in antioxidant GSH and the increase in lipid peroxidation observed in brain tissue during obstructive apneas—but not during the intermittent hypoxia—suggest that the response of increased PtO2 in brain tissue occurs at expenses of generating oxidative stress.
The experimental setting for applying obstructive apneas was based on a previously reported experimental model of OSA13 that mimicked the recurrent asphyxia and inspiratory efforts in severe OSA patients. In contrast, the intermittent hypoxia protocol used in this work was chosen to replicate only the cyclical arterial desaturations of the obstructive apnea protocol. The differences observed when the animals were subjected to both stimuli suggest that the use of obstructive apneas appears to be more adequate than the more commonly used setting of intermittent hypoxia to investigate the mechanisms involved in the brain response to OSA.
The increase in cerebral cortex PtO2 during recurrent obstructive apneas suggests the presence of a number of compensatory mechanisms that increase the availability of oxygen in cerebral tissue in response to this stimulus, but not during application of intermittent hypoxia. This differential response could be due to the concomitant hypercapnia generated during the recurrent asphyxia in those animals undergoing the obstructive apnea protocol. A high level of CO2 has been suggested that could modulate brain tissue oxygenation during airway occlusion.17 Indeed, hypercapnia induces a higher delivery of oxygen from erythrocytes,18 and by inducing vasodilation,19 it promotes adaptive changes in cerebral hemodynamics that increase cerebral blood flow17,20,21 and can cause hyperperfusion.22 In addition to hypercapnia, other mechanisms might contribute to adaptive changes to obstructive apneas: for instance, reduced mitochondrial activity in neuronal cells23 and activation of the transcription factor hypoxia inducible factor (HIF-1) that up-regulates the expression of target genes, including vascular endothelial growth factor (VEGF), erythropoietin (EPO), and nitric oxide synthase (NOS).24–26 Nevertheless, the latter changes might require longer than the time frame of our current study. Overall, the time-course response of brain tissue PtO2 during obstructive apneas could be the result of multiple adjustments—involving increased cerebral perfusion, blood oxygen supply, and a decrease in oxygen consumption in brain tissue—that are regulated by complex mechanisms.
Mean cerebral cortex PtO2 during intermittent hypoxia tended to be lower than baseline, although it increased during recurrent obstructive apneas (Figure 2B). The greater availability of oxygen in brain tissue during obstructive apneas might prevent the type of neuronal death induced by low levels of oxygen tension,7,8 but it might also induce neuronal suffering caused by increased oxidative stress. A priori, oxidative stress could occur in response to both stimuli (obstructive apneas and intermittent hypoxia) since the PtO2 oscillations experienced were similar in both groups. We observed that VEGF protein levels were similar after both types of stimuli, probably due to the early time point of sampling, only one hour after the onset of obstructive apneas or intermittent hypoxia. Nevertheless, we observed a strong decay in the content of reduced glutathione (GSH) after the obstructive apnea challenge alone. GSH is the most abundant non-protein thiol buffering reactive oxygen species in brain tissue.27 Although both continuous and intermittent hypoxia could promote oxidative stress in brain tissue,10 the increased level of PtO2 experienced in cerebral cortex during obstructive apneas with PtO2 oscillations might contribute to the promotion of oxidative stress. Indeed, increased oxygen levels can induce the production of free radicals and promote the consumption of natural antioxidant reservoirs, such as GSH. In support of this, lipid peroxidation significantly increased in brain tissue after obstructive apneas, while only a trend was observed after intermittent hypoxia, providing evidence for a higher degree of oxidative stress under the former condition. A recent study with a model of transient focal cerebral ischemia found the worst brain injury and neurological deficits in animals developing hyperemia at the time of reperfusion.16 The authors reported that treatment with antioxidants attenuated the consumption of GSH induced by ischemia and was more beneficial in hyperemic than in non-hyperemic rats, suggesting a relationship between hyperemia and oxidative stress.16 Similarly, prior administration of green tea catechin polyphenols in rats attenuates the behavioral and oxidative stress response induced by chronic intermittent hypoxia.28 Although animals subjected to obstructive apneas presented a rapid increase in oxygen levels that preserve brain tissue against repeated hypoxic periods, this greater supply of oxygen could be responsible for the rapid induction of oxidative stress observed after 1 h of obstructive apneas. In contrast, the rats subjected to intermittent hypoxia presented lower levels of oxygen in cerebral cortex, and no changes in GSH were observed. Contrary to the case of brain tissue, no differences were found in skeletal muscle PtO2 when comparing obstructive apneas and intermittent hypoxia. The patterns of maximum and minimum values attained by hypoxic events in both protocols were similar to those observed in SpO2 (Figure 2). These results provide new insights into PtO2 behavior in skeletal muscle during obstructive apneas, as previous data were recorded only in response to intermittent hypoxia.29 Interestingly, the PtO2 response observed in visceral fat tissue was similar to that found in skeletal muscle. Therefore, both skeletal muscle and visceral fat tissues were under hypoxic conditions with respect to baseline during either recurrent obstructive apneas or intermittent hypoxia, and no evidence for increased oxidative stress was found in these tissues after 1 h of challenge. However, the hypoxic condition of fat tissue could worsen the local hypoxic environment under obesity, a common condition in OSA patients, since it is associated with adipocyte hypertrophy and enhanced adipokine and leptin production.30 Overall, the PtO2 patterns experienced by all the tissues investigated suggest a selective increase of oxygen supply for apneic events in cerebral cortex tissue, but not in skeletal muscle and visceral fat, which is also supported by a rapid induction of oxidative stress and consumption of GSH antioxidant in cerebral cortex compared to the other tissues. These findings could be explained in part by a preferential redistribution of blood flow to essential organs after arterial chemoreceptors are activated by hypoxemia.31,32
Our findings were taken from young healthy animals to isolate the effects of obstructive apneas or intermittent hypoxia stimuli on tissue oxygenation. Accordingly, direct translation of the results to patients with OSA would require further investigation in animals that mimic more closely the pathophysiology of the syndrome. For instance, OSA is commonly associated with aging, obesity, and hypertension, which can promote endothelial dysfunction33; and chronic exposure to OSA may affect cerebral circulation.34 Taking into account the fact that hemodynamic changes induced by obstructive apneas could be affected by age35 and endothelial dysfunction,36 the PtO2 behavior observed in the cerebral cortex of young healthy animals could be compromised by these coexistent factors. Therefore, future studies focusing on the PtO2 response to apneas in aged, obese, diabetic, or hypertensive groups of animals would be of great interest in ascertaining how each of these factors affects brain tissue oxygenation and oxidative stress in patients with OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Miguel A. Rodríguez, Noelia Montoya, and Leonardo Márquez for their help in the laboratory procedures. This work was supported in part by Ministerio de Ciencia e Innovación (SAF2009-2991, PI08/0277, PI081908), SEPAR and FUCAP.
REFERENCES
- 1.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Prabhakar NR, Kumar GK. Oxidative stress in the systemic and cellular responses to intermittent hypoxia. Biol Chem. 2004;385:217–21. doi: 10.1515/BC.2004.015. [DOI] [PubMed] [Google Scholar]
- 4.Atkeson A, Jelic S. Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag. 2008;4:1327–35. doi: 10.2147/vhrm.s4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banasiak KJ, Haddad GG. Hypoxia-induced apoptosis: Effect of hypoxic severity and role of p53 in neuronal cell death. Brain Res. 1998;797:295–304. doi: 10.1016/s0006-8993(98)00286-8. [DOI] [PubMed] [Google Scholar]
- 6.Bossenmeyer-Pourie C, Koziel V, Daval JL. CPP32/CASPASE-3-like proteases in hypoxia-induced apoptosis in developing brain neurons. Brain Res Mol Brain Res. 1999;71:225–37. doi: 10.1016/s0169-328x(99)00190-4. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Chi L, Row BW, et al. Increased oxidative stress is associated with chronic intermittent hypoxia-mediated brain cortical neuronal cell apoptosis in a mouse model of sleep APNEA. Neuroscience. 2004;126:313–23. doi: 10.1016/j.neuroscience.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Zhang JH, Fung SJ, Xi M, Sampogna S, Chase MH. Recurrent apnea induces neuronal apoptosis in the guinea pig forebrain. Exp Neurol. 2009;216:290–4. doi: 10.1016/j.expneurol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Nagata N, Saji M, Ito T, Ikeno S, Takahashi H, Terakawa N. Repetitive intermittent hypoxia-ischemia and brain damage in neonatal rats. Brain Dev. 2000;22:315–20. doi: 10.1016/s0387-7604(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 10.Row BW, Liu R, Xu W, Kheirandish L, Gozal D. Intermittent hypoxia is associated with oxidative stress and spatial learning deficits in the rat. Am J Respir Crit Care Med. 2003;167:1548–53. doi: 10.1164/rccm.200209-1050OC. [DOI] [PubMed] [Google Scholar]
- 11.Almendros I, Montserrat JM, Torres M, Gonzalez C, Navajas D, Farre R. Changes in oxygen partial pressure of brain tissue in an animal model of obstructive apnea. Respir Res. 2010;11:3. doi: 10.1186/1465-9921-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004;75:353–61. doi: 10.1136/jnnp.2003.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carreras A, Almendros I, Acerbi I, Montserrat JM, Navajas D, Farre R. Obstructive apneas induce early release of mesenchymal stem cells into circulating blood. Sleep. 2009;32:117–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher EC, Costarangos C, Miller T. The rate of fall of arterial oxyhemoglobin saturation in obstructive sleep-apnea. Chest. 1989;96:717–22. doi: 10.1378/chest.96.4.717. [DOI] [PubMed] [Google Scholar]
- 15.Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990;190:360–5. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Asensio FJ, de la Rosa X, Jimenez-Altayo F, et al. Antioxidant CR-6 protects against reperfusion injury after a transient episode of focal brain ischemia in rats. J Cereb Blood Flow Metab. 2010;30:638–52. doi: 10.1038/jcbfm.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brzecka A. Role of hypercapnia in brain oxygenation in sleep-disordered breathing. Acta Neurobiol Exp (Wars) 2007;67:197–206. doi: 10.55782/ane-2007-1648. [DOI] [PubMed] [Google Scholar]
- 18.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol. 2009;212:3387–93. doi: 10.1242/jeb.023697. [DOI] [PubMed] [Google Scholar]
- 19.Van de Ven MJ, Colier WN, Van der Sluijs MC, Kersten BT, Oeseburg B, Folgering H. Ventilatory and cerebrovascular responses in normocapnic and hypercapnic COPD patients. Eur Respir J. 2001;18:61–8. doi: 10.1183/09031936.01.00087501. [DOI] [PubMed] [Google Scholar]
- 20.Levasseur JE, Wei EP, Kontos HA, Patterson JL., Jr. Responses of pial arterioles after prolonged hypercapnia and hypoxia in the awake rabbit. J Appl Physiol. 1979;46:89–95. doi: 10.1152/jappl.1979.46.1.89. [DOI] [PubMed] [Google Scholar]
- 21.Olsen AK, Keiding S, Munk OL. Effect of hypercapnia on cerebral blood flow and blood volume in pigs studied by positron emission tomography. Comp Med. 2006;56:416–20. [PubMed] [Google Scholar]
- 22.Pollock JM, Deibler AR, Whitlow CT, et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am J Neuroradiol. 2009;30:378–85. doi: 10.3174/ajnr.A1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makarov PR, Wiswedel I, Augustin W, Schild L. Hypoxia/reoxygenation-induced damage to mitochondrial activity is determined by glutathione threshold in astroglia-rich cell cultures. Brain Res. 2002;933:91–7. doi: 10.1016/s0006-8993(02)02246-1. [DOI] [PubMed] [Google Scholar]
- 24.Sharp FR, Ran R, Lu A, et al. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx. 2004;1:26–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–9. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 26.Ran RQ, Xu HC, Lu AG, Bernaudin M, Sharp FR. Hypoxia preconditioning in the brain. Dev Neurosci. 2005;27:87–92. doi: 10.1159/000085979. [DOI] [PubMed] [Google Scholar]
- 27.Dringen R, Gutterer JM, Hirrlinger J. Glutathione metabolism in brain - Metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem. 2000;267:4912–6. doi: 10.1046/j.1432-1327.2000.01597.x. [DOI] [PubMed] [Google Scholar]
- 28.Burckhardt IC, Gozal D, Dayyat E, et al. Green tea catechin polyphenols attenuate behavioral and oxidative responses to intermittent hypoxia. Am J Respir Crit Care Med. 2008;177:1135–41. doi: 10.1164/rccm.200701-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aravindan N, Aravindan S, Shanmugasundaram K, Shaw AD. Periods of systemic partial hypoxia induces apoptosis and inflammation in rat skeletal muscle. Mol Cell Biochem. 2007;302:51–8. doi: 10.1007/s11010-007-9424-7. [DOI] [PubMed] [Google Scholar]
- 30.Wood I, Heredia F, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–7. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 31.Lou HC, Tweed WA, Davies JM. Preferential blood-flow increase to the brain-stem in moderate neonatal hypoxia - reversal by naloxone. Eur J Pediatr. 1985;144:225–7. doi: 10.1007/BF00451945. [DOI] [PubMed] [Google Scholar]
- 32.Schultz HD, Li YL, Ding YF. Arterial chemoreceptors and sympathetic nerve activity-Implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 33.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci. 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan B, Reichmunth K, Peppard P, et al. Effect of sleep disordered breathing on cerebrovascular regulation: A population-based study. Am J Respir Crit Care Med. 2010;182:1445–52. doi: 10.1164/rccm.201002-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatto R, Hoffman WE, Mueller M, Paisansathan C, Charbel F. Age effects on brain oxygenation during hypercapnia. J Biomed Opt. 2007;12:062113. doi: 10.1117/1.2804705. [DOI] [PubMed] [Google Scholar]
- 36.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–10. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]