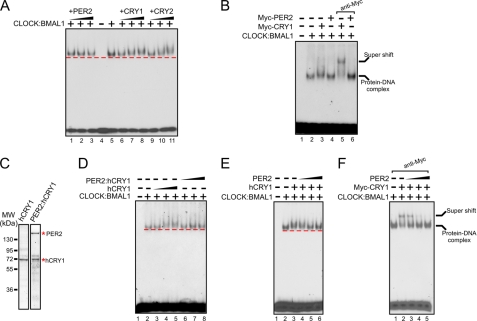
FIGURE 3.
Effect of PER on clock protein-DNA complexes. A, effect of PER2 on the CLOCK:BMAL1:E-box complex. The 14-bp E-box duplex (1 nm) was incubated with CLOCK:BMAL1 (0.2 nm) and then increasing concentrations (0.2, 0.6, and 1.5 nm) of PER2, CRY1, or CRY2 were added, and the protein-DNA complexes were analyzed by EMSA. The dashed line indicates the leading edge of migration of the CLOCK:BMAL1:E-box complex. Note the lack of effect of PER2 on the mobility of this complex (lanes 1–3). In contrast both CRY1 (lanes 6–8) and CRY2 (lanes 9–11) retard the DNA-protein complex. B, analysis of the composition of the protein-DNA bands by antibody supershift. In addition to CLOCK:BMAL1 (0.2 nm) equal concentrations of either Myc-PER2 (1 nm) or Myc-CRY1 (1 nm) were included in the reactions followed by anti-Myc antibodies in the last two lanes. Note the supershift of the protein-DNA band formed in the presence of Myc-CRY1 (lane 5) but not Myc-PER2 (lane 6). C, Coomassie Blue staining of SDS-PAGE showing the hCRY1 and PER2:hCRY1 used in the electrophoretic mobility shift assay. D, effects of hCRY1 and PER2:hCRY1 on CLOCK:BMAL1:E-box complexes. Increasing concentrations (0.1, 0.3, and 1 nm) of hCRY1 or the PER2:hCRY1 complex were added to the reactions as indicated before electrophoresis. Note the supershift caused by hCRY1 (lanes 4 and 5) but not by the PER2:hCRY1 complex (lanes 6–8) containing the same concentrations of hCRY1 as in lanes with hCRY1 alone. E and F, PER2 removes CRY1 from the CRY1:CLOCK:BMAL1:E-box complex. The E-box duplex was incubated with the indicated proteins: 0.2 nm CLOCK:BMAL1 dimer and 0.3 nm hCRY1 (in panel E) or 0.3 nm Myc-CRY1 (in panel F) before separating on a native gel. Note that the presence of hCRY1 causes a slight retardation of the CLOCK:BMAL1:E-box complex (panel E, lane 3), which is reversed by inclusion of increasing concentrations (0.2, 0.6, and 1.2 nm) of PER2 (panel E, lanes 4–6). The effect of PER2 on removing CRY1 from the protein-DNA complex is more clearly shown when the anti-Myc antibody supershifted Myc-CRY1:CLOCK:BMAL1:E-box complex is incubated with increasing concentrations of PER2 (panel F, lanes 3–5).