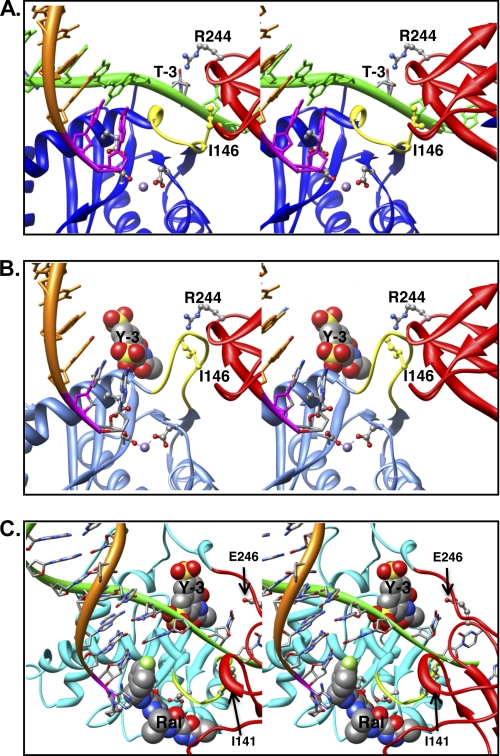
FIGURE 7.
Model of IN bound to a viral DNA end in the absence or presence of the inhibitors. A, the model of ASV IN was prepared using crystal structure of PFV IN bound to viral DNA as a template (Protein Data Bank ID code 3L2Q) (31, 40) and is portrayed as a stereo pair. The cleaved strand is shown as an orange ribbon, with the conserved CA residues in magenta. The noncleaved strand is in green and is held by cooperation of the CTD (red ribbon) and the flexible loop (yellow ribbon) adjacent to the active site in the CCD (blue ribbon). For clarity, the linkers between the NTD, core, and CTD domains are not shown. Active site residues that bind the divalent metal cofactors (purple sphere) as well as the two residues proposed to participate in positioning the noncleaved strand are portrayed in ball and stick: Ile146 (yellow) in the flexible loop and Arg244 in the CTD. The PFV residue Asn348, which is analogous to ASV Arg244, has been shown to make base-specific contacts; the Arg244 rotamer shown is in a position to make similar contact with the thymine at the −3 position in the noncleaved viral DNA strand. B, model of ASV IN bound to inhibitor Y-3. Color code is the same as in A except that the core domain ribbon is now shown in light blue. The Y-3 inhibitor is shown in space-filling representation in its previously established binding site (32), which induces a change in the position of the flexible loop and Ile146 (yellow) that preclude proper binding of the noncleaved DNA strand. C, model of HIV-1 IN bound to both Y-3 and raltegravir. Colors are as above, except that the HIV-1 CCD is in cyan. To position Y-3, the HIV-1 intasome model with bound raltegravir (21) was superimposed onto the ASV IN catalytic core bound to Y-3. As with ASV IN, Y-3 is predicted to interfere sterically with binding of the noncleaved strand of HIV viral DNA. In contrast, the presence of viral DNA in the complex enhances raltegravir binding to HIV-1 IN, but such binding is predicted to interfere with target DNA binding. Importantly, Y-3 and raltegravir are predicted to bind on opposite sides of the flexible loop.