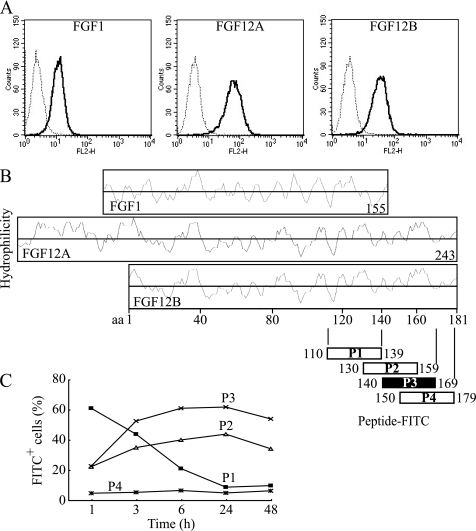
FIGURE 3.
C-terminal amino acid sequence of FGF12 involved in cellular internalization. A, human recombinant FGF1, FGF12A, and FGF12B (R&D) were labeled with Alexa Fluor 568. FGF1 and FGF12B had no tag, whereas FGF12A had a 3xFLAG-His6 tag. IEC6 cells were incubated with 1 μg/ml of each Alexa Fluor 568-labeled FGF for 24 h. The cells were harvested in trypsin-EDTA, washed twice with PBS containing 0.2% BSA, and subjected to FACS Calibur flow cytometry to estimate fluorescence intensity. A dotted line shows the control cells, and a solid line shows the FGF-treated cells. B, each Hopp-Woods scale, hydrophilicity plot, for FGF1, FGF12A, and FGF12B was compared with identify the possible domains of FGFs. Four peptides containing 30 amino acid residues conjugated with FITC were synthesized on the basis of the C-terminal sequence of FGF12, and then purified to more than 95% purity using HPLC as specified by the manufacturer (Invitrogen). C, kinetics of cellular uptake of FITC-labeled peptides were examined over 48 h by FACS. IEC6 cells were cultured with 10 μg/ml of each FITC-labeled peptide and subjected to flow cytometry to assess the level of cellular uptake of each peptide.