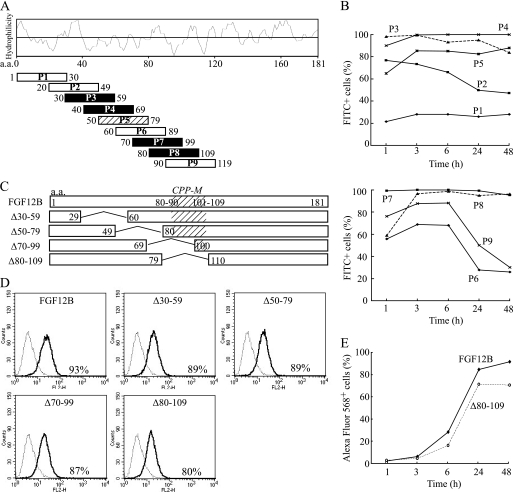
FIGURE 5.
Possible involvement of an internal region of FGF12 in cellular internalization. A, each 30 amino acid peptide was synthesized on the basis of the N-terminal and internal FGF12B sequence and conjugated with FITC. A Hopp-Woods scale, hydrophilicity plot for FGF12B, is shown to compare the hydrophilicity of all peptides. Black squares indicated the most likely CPPs on the basis of FACS analysis and a slash square is the next most likely. B, kinetics of cellular uptake of FITC-labeled peptides were examined over 48 h by flow cytometry. IEC6 cells were cultured with 10 μg/ml of each FITC-labeled peptide and subjected to flow cytometry to assess the level of cellular uptake of each peptide. C, each of the amino acid residues 30–59, 50–79, 70–99, and 80–109 of FGF12B were deleted from the FGF12B protein to create FGF12B mutants (Δ30–59, Δ50–79, Δ70–99, and Δ80–109, respectively). As a result, FGF12B and mutant proteins were fused to a Nus tag. D, IEC6 cells were incubated in complete medium with 1 μg/ml Alexa Fluor 568-labeled FGF12 mutant proteins for 24 h. They were analyzed by flow cytometry to estimate fluorescence intensity. E, kinetics of cellular uptake of Alexa Fluor 568-labeled wild-type FGF12B and Δ80–109 were examined over 48 h by FACS.