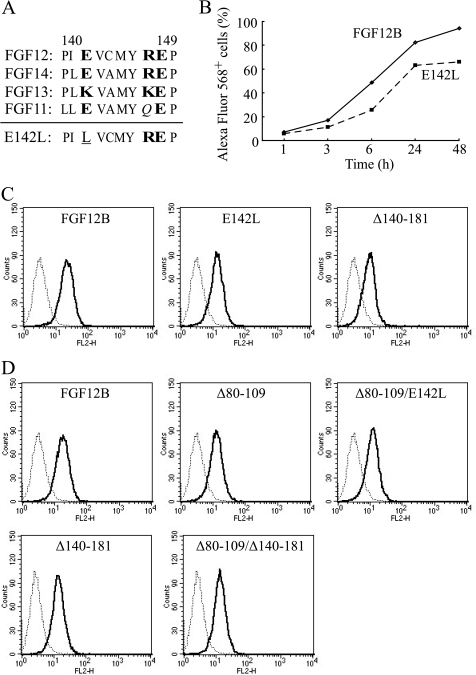
FIGURE 6.
Decrease in FGF12 internalization through a single mutation in the C-terminal region of FGF12. A, amino acid sequences of the FGF11 subfamily corresponding to amino acids 140–149 (CPP-C) of FGF12B are shown. Plain letters indicate hydrophobic amino acids. Hydrophilic amino acid residues are printed in bold letters and neutral amino acid residues are in italics. A single substitution, E142L, was introduced into the CPP-C domain, shown underlined, to examine the contribution of CPP-C to cellular uptake. B, kinetics of cellular uptake of Alexa Fluor 568-labeled wild-type FGF12B and FGF12B mutant E142L were examined over 48 h by FACS. C, representative FACS histograms are shown in red-orange fluorescence Alexa 568-positive cell populations after 24 h culture with 1 μg/ml of FGF12B, E142L, and Δ140–181. D, single substitution E142L and C-terminal deletion of residues 140–181 were made in the Δ80–109 proteins (Δ80–109/E142L and Δ80–109/Δ140–181, respectively) to examine any additive effect of CPP-M and CPP-C on cellular internalization. Each recombinant protein was fused to a Nus tag. IEC6 cells were incubated in complete medium with 1 μg/ml Alexa Fluor 568-labeled FGFs for 24 h. The cells were analyzed by flow cytometry to estimate fluorescence intensity.