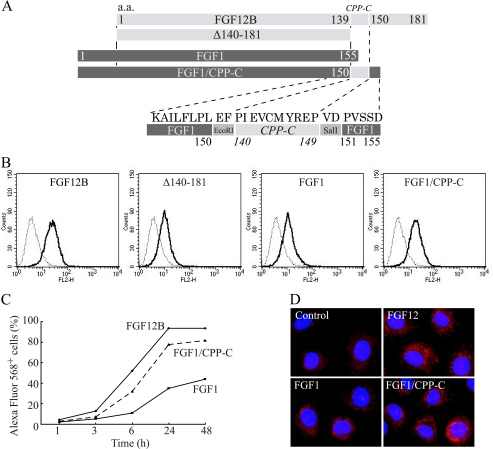
FIGURE 7.
Enhancement of FGF1 internalization by CPP-C. A, amino acid residues 140–149 (CPP-C) of FGF12B were fused to the C-terminal end of the human FGF1 sequence to create an FGF1/CPP-C fusion protein (FGF1/CPP-C) to examine the capability of CPP-C to deliver FGF1 into cells. B, IEC6 cells were incubated in complete medium with 1 μg/ml Alexa Fluor 568-labeled FGF12B, Δ140–181, FGF1, or FGF1/CPP-C for 24 h. The cells were analyzed by flow cytometry to estimate fluorescence intensity. C, kinetics of cellular uptake of FGF12B, FGF1, and FGF1/CPP-C were examined over 48 h by FACS. D, IEC6 cells were cultured for 18 h in medium with 1 μg/ml Alexa Fluor 568-labeled FGF12, FGF1, or FGF1/CPP-C. The nuclei were visualized by staining with Hoechst 33342 (blue) and fluorescence confocal images were acquired using an IX81 fluorescence microscope with disk-scanning unit (DSU) (Olympus, Tokyo, Japan).