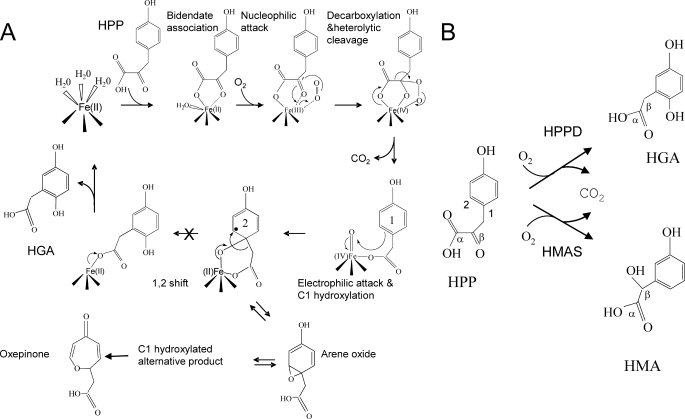
FIGURE 1.
A, proposed mechanism of the reaction catalyzed by HPPD. When the last 1,2 rearrangement is blocked (S246A HPPD mutant), an arene oxide-derived intermediate is released as an alternative product. This compound presents very similar chemical properties than the intermediate observed by Gunsior et al. (17) with their S. avermitilis P214T HPPD mutant, which they identified by NMR after HPLC purification as an oxepinone. B, comparison of the reactions catalyzed by HPPD and HMAS. 1, enzyme-HPA-FeIV-oxene intermediate; 2, enzyme-C1-hydroxylated intermediate with a radical σ complex (black dot).