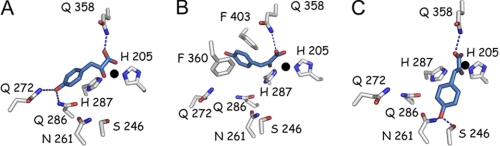
FIGURE 2.
Three different models of HPP binding within the Arabidopsis HPPD active site (1SQD). A, binding model proposed by Serre et al. (20) consistent with that observed for other α-keto acid-dependent enzymes. This binding mode was reliant upon the following: (a) coordination of the iron metal ion by the HPP α-keto acid moiety (bidentate) and side chains of amino acids His-205, His-287, and Glu-373 within the active site (Glu-373 not shown for clarity), and (b) hydrogen bonding of HPP to conserved glutamine residues (Gln-272, Gln-286, and Gln-358). B, binding model proposed by Brownlee et al. (23), consistent with the crystallographically observed binding position of a structurally related inhibitor 2-[2-nitro-4-(trifluoromethyl)benzoyl]-1,3 cyclohexanedione in complex with HPPD (23). In this proposed binding model, the HPP α-keto acid moiety still makes bidentate contact with the metal ion and hydrogen bond with a conserved glutamine residue (Gln-358). However, its 4-hydroxyl group is no longer engaged in hydrogen bonding with amide side chains of conserved residues but is involved in π-stacking with the rings of two conserved phenylalanine residues (Phe-360 and Phe-403). C, HPP-binding position based on the structure of HMAS Co(II)-HMA complex (2R5V) (38). In this position, the HPP α-keto acid moiety again makes bidentate contact with the metal ion and hydrogen bond with a conserved glutamine residue (Gln-358). However, the 4-hydroxyl group is engaged in hydrogen bonding with Ser-246 and Asn-261. HPP is colored blue, and the active site iron is represented as a black sphere.