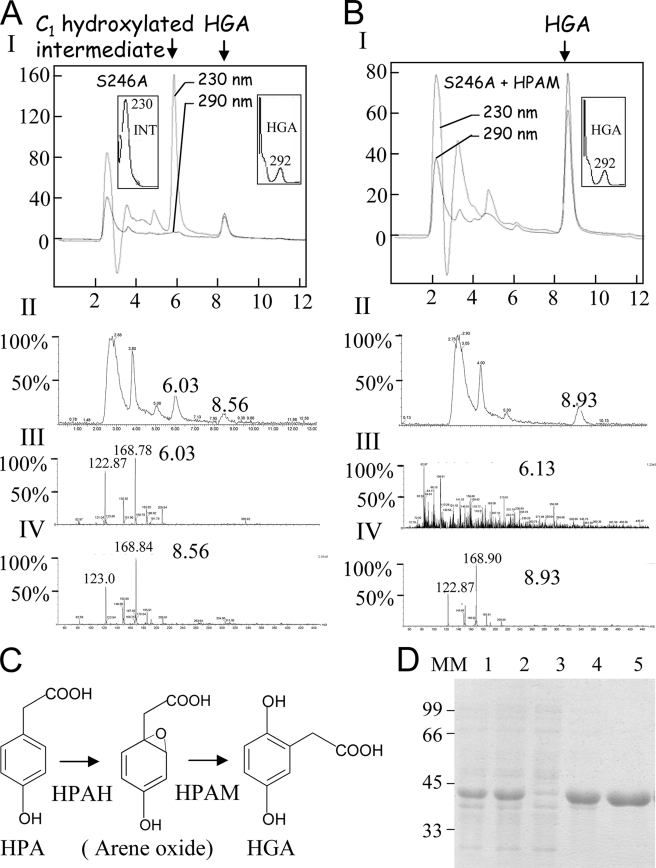
FIGURE 4.
Analyses of the intermediate released by the S246A mutant. A, LC/MS analysis. Panel I, HPLC recorded at 230 and 290 nm of 50 μl of S246A reaction medium. The absorption spectrum of the C1-hydroxylated intermediate and HGA are presented in the left and right insets, respectively. Panel II, MS chromatogram. Panel III, LC/MS spectrum of the elution pick at 6.03 min (C1-hydroxylated intermediate). Panel IV, LC/MS spectrum of the elution pick at 8.5 min (HGA). Note that although the two products have different retention times and absorption spectra, they have the same m/z of 169. B, C1-hydroxylated intermediate released by the S246A mutant is transformed into HGA by HPAM. Panel I, HPLC recorded at 230 and 290 nm after incubation of the S246A mutant reaction medium with HPAM (injected volume 25 μl). Absorption spectrum of HGA is presented in the inset. Note that all the C1-hydroxylated intermediate was transformed into HGA. Panel II, MS chromatogram. Panel III, LC/MS spectrum of the elution pick at 6.13 min (C1-hydroxylated intermediate). Panel IV, LC/MS spectrum of the elution pick at 6.13 min (HGA). Note that no product could be detected at 6.3 min. C, schematic representation of the reaction catalyzed by the P. acidovorans 4-HPA-1-hydroxylase two-enzyme system constituted by an HPAH and a new HPAM. D, purification of recombinant P. acidovorans HPAM by Ni-NTA affinity column. Lane 1, 50 μg of total protein extract; lane 2, 50 μg of soluble protein extract; lane 3, run off; lane 4, wash with 50 mm imidazole; lane 5, elution with 250 mm imidazole. MM, molecular mass. Arene oxide, proposed C1-hydroxylated product released by HPAH.