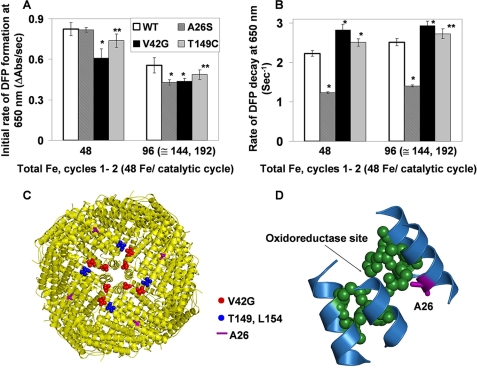
FIGURE 4.
Ala26 near the active site destabilizes the DFP catalytic intermediates in ferritin. Single turnovers (48 Fe2+/nanocage; 2 Fe2+/oxidoreducatse site) by wild-type frog-M ferritin (WT) and site-directed amino acid substitutions variants were monitored, ΔA650 nm (DFP) and ΔA350 nm (Fe3+O, which includes DFP, diferric oxo, Fe3+O tetramers, and mineral), after rapidly (<5 ms) mixing equal volumes ferritin cage protein with ferrous sulfate solutions, described in Fig. 2 and under “Experimental Procedures.” The results are averages from two to three preparations of protein analyzed four to six times each, and the error is the S.D. Significantly different from WT, *, p < 0.0001 or **, p < 0.006 (t test). A, initial rates of DFP formation (ΔA650 nm/s). B, initial rates of DFP decay. C, frog-M ferritin nanocage structure viewed from the outside, with the 4-fold axis near the center (Protein Data Bank ID code 1MFR). The residues near the 4-fold pores are shown in red or blue spheres. D, part of a single subunit of the frog-M ferritin showing the ferroxidase center and the position of residue Ala26 nearby.