Abstract
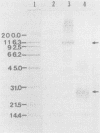
A cDNA clone derived from sporulated oocysts of Eimeria tenella and encoding the expression product GX3262 was identified using a monoclonal antibody raised against Eimeria acervulina sporozoites. The cDNA fragment containing the coccidial antigen gene was cloned in bacteriophage lambda gt11, transferred to a plasmid, and introduced into Escherichia coli for analysis of the gene products. The strain carrying the plasmid produced GX3262 as part of a fusion protein consisting of the first 1,006 amino acids of E. coli beta-galactosidase and 112 amino acids of the E. tenella protein of approximately 12 kilodaltons. Partially purified antigen, heat-killed recombinant bacterin, and live E. coli containing the recombinant coccidial antigen were used to immunize 1-week-old or newly hatched broiler chicks. Several immunization protocols were utilized, including boosts with partially purified beta-galactosidase-GX3262, bacterin, or small numbers of live E. tenella oocysts. After challenge with an experimental E. tenella infection, the birds were evaluated by scoring cecal lesions to determine the level of protection. The greatest degree of protection was seen after only a single immunization of 2-day-old birds with a live recombinant E. coli preparation. The results presented here identify GX3262 as a potential candidate coccidial vaccine antigen and provide evidence for the first time that newly hatched chickens can be successfully vaccinated with a recombinant antigen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Danforth H. D., Augustine P. C. Specificity and crossreactivity of immune serum and hybridoma antibodies to various species of avian coccidia. Poult Sci. 1983 Nov;62(11):2145–2151. doi: 10.3382/ps.0622145. [DOI] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W. M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970 Aug;28(1):30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long P. L. Modulation of coccidial infections by drugs. Prog Clin Biol Res. 1984;161:213–219. [PubMed] [Google Scholar]
- Long P. L. The effect of breed of chickens on resistance to Eimeria infections. Br Poult Sci. 1968 Jan;9(1):71–78. doi: 10.1080/00071666808415695. [DOI] [PubMed] [Google Scholar]
- Pasternak J., Winkfein R. J., Fernando M. A. Ribosomal and translatable messenger RNA of Eimeria tenella. Mol Biochem Parasitol. 1981 Jul;3(3):133–142. doi: 10.1016/0166-6851(81)90044-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Tam J. P., Hollingdale M. R., Cochrane A. H., Quakyi I., Nussenzweig R. S., Nussenzweig V. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985 Jun 21;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]