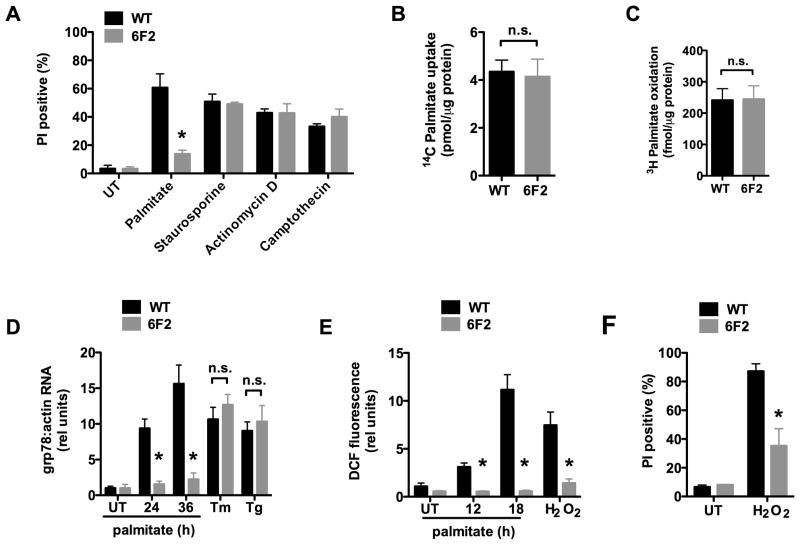
Figure 1. 6F2 cells are resistant to palmitate-induced lipotoxicity.
(A) Wild type (WT) and mutant 6F2 CHO cells were untreated (UT) or supplemented with 500 μM palmitate for 48h or with 80 nM staurosporine, 2 μM actinomycin D, or 10 μM camptothecin for 24 h. Cell death was quantified by propidium iodide (PI) staining and flow cytometry on 104 cells/sample. (B) WT and 6F2 cells were incubated with 14C-palmitate for 1 min. Uptake of labeled palmitate was assessed by scintillation counting. (C) WT and 6F2 cells were incubated with 3H-palmitate for 2 h. 3H2O production as a measure of fatty acid oxidation was assessed by scintillation counting. (D) WT and 6F2 cells were treated with palmitate for indicated times, or with the ER stress inducers, tunicamycin (Tm, 2.5 μg/ml) or thapsigargin (Tg, 1 μM) for 6 h. grp78 expression was analyzed by quantitative real-time PCR (qRT-PCR) and normalized to β-actin expression. (E) WT and 6F2 cells were treated with palmitate for indicated times or with 500 μM H2O2 for 1 h. ROS generation was quantified by CM-H2DCFDA (DCF) labeling and flow cytometry on 104 cells/sample. (F) WT and 6F2 cells were UT or treated with 2.3 mM H2O2 for 20 h. Cell death was quantified by PI staining and flow cytometry on 104 cells/sample. All data expressed as mean ± SE for 3 independent experiments. * p < 0.01 for mutant 6F2 vs. WT; n.s., not significant. (See also supplemental Figure 1.)