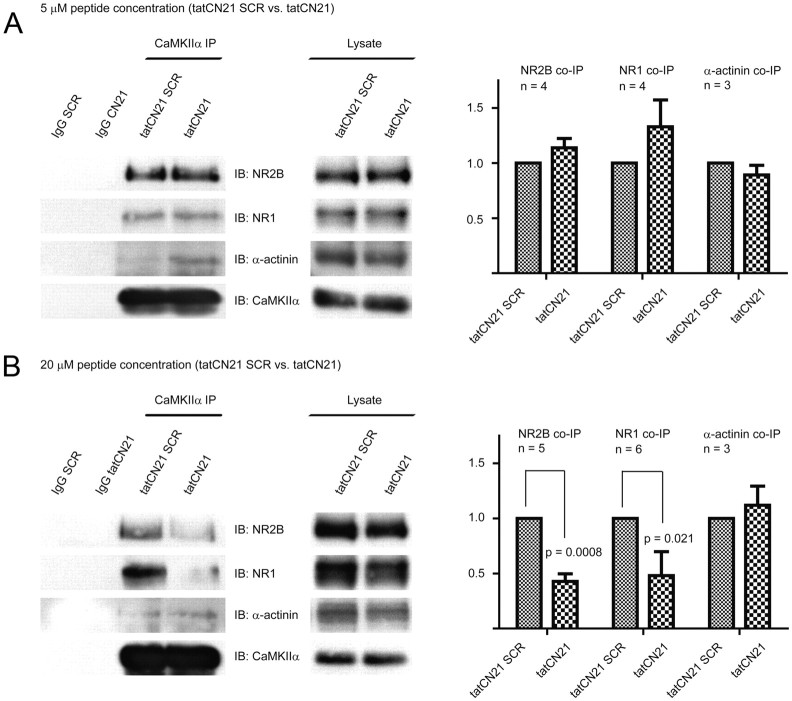
Figure 6.
TatCN21 persistently disrupts the CaMKII/NMDAR complex at 20 μm, but not 5 μm. After the same preincubation procedure described in Figure 5, slices were frozen for biochemical analysis. The NMDA receptor complex was solubilized with 1% deoxycholate before IP with control IgG or CaMKIIα antibody and IB for NR2B, NR1, α-actinin, and CaMKIIα. The immunosignals were quantified by densitometry, NMDAR and α-actinin signals were divided by CaMKIIα signals to correct for any variability in CaMKII IP, and tatCN21 values were normalized to SCR values (100%). Bars, Averages ± SEM. A, Five micromolar tatCN21 for 1 h does not affect basal CaMKII interaction with NMDAR (NR2B: CN21/SCR = 1.177 ± 0.076, p = 0.199, n = 4; NR1: CN21/SCR = 1.399 ± 0.135, p = 0.266, n = 4; averages ± SEM, paired t test) or α-actinin (CN21/SCR = 0.892 ± 0.088, n = 3). B, Twenty micromolar tatCN21 for 2 h produces persistent reduction of basal CaMKII interaction with NMDAR (NR2B: CN21/SCR = 0.386 ± 0.068, p = 0.0008, n = 5; NR1: CN21/SCR = 0.508 ± 0.156, p = 0.021, n = 6), but not α-actinin (CN21/SCR = 1.083 ± 0.189, n = 3).