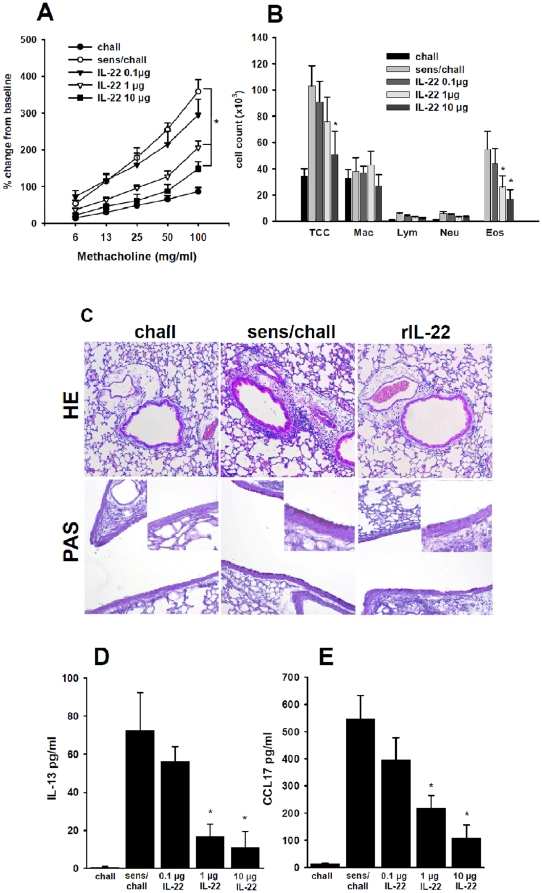
Figure 8. Administration of rIL-22 reduces AHR and airway inflammation.
Airway responsiveness (panel A), cell counts in BAL fluid (panel B) and lung tissue inflammation and goblet cell metaplasia (panel C) were assessed in mice 48 h after the last airway challenge. Mice which were sensitized and challenged (sens/chall, n = 12) showed increased airway reactivity and numbers of eosinophils in BAL fluid compared to challenged only mice (chall, n = 5), where no eosinophils were detectable. Intranasal treatment of sensitized and challenge animals with 0.1 µg recombinant IL-22 (IL-22 0.1 µg, n = 12) showed little effects on AHR and inflammation. In contrast, mice treated with either 1 µg (IL-22 1 µg, n = 12) or 10 µg (IL-22 1 µg, n = 12) of recombinant IL-22 showed decreased AHR and number of eosinophils in BAL fluid. Means±SEM are given, *p<0.05 compared to sens/chall. Panel C: Tissue inflammation was evaluated 48 hrs following the last challenge using hematoxylin and eosin staining (HE) and PAS staining for goblet cells in challenged only mice (chall), non-treated sensitized and challenged mice (sens/chall) and sensitized and challenged animals treated with 10 µg of recombinant IL-22 (rIL-22). Final magnifications 100× and 400× for inserts. Panels D and E: Levels of IL-13 (panel D) and CCL17 (panel E) were measured in BAL fluid by ELISA 48 h after the last challenge. Means±SEM of challenged only mice (chall, n = 5), non-treated sensitized and challenged mice (sens/chall, n = 12), and sensitized and challenged mice treated with 0.1 µg (0.1 µg IL-22, n = 12), 1 µg (1 µg IL-22, n = 12) and 10 µg (10 µg IL-22, n = 12) of rIl-22, respectively. Mean±SEM are given. * p<0.05 compared to sens/chall and 0.1 µg IL-22.