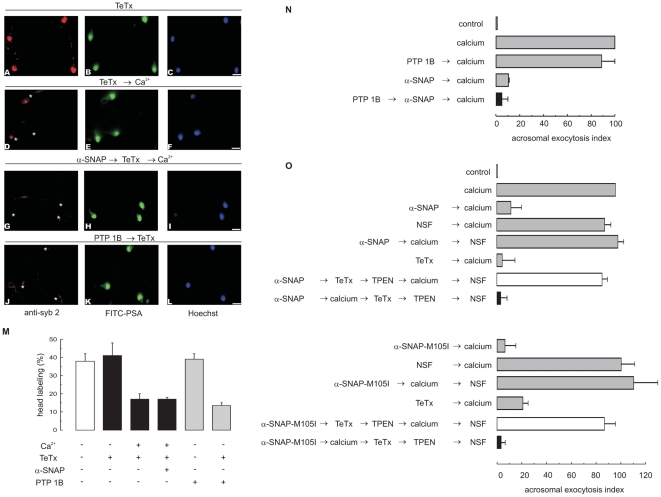
Figure 7. Recombinant α-SNAP halts the AR at a stage when SNAREs are monomeric.
A, we incubated SLO-permeabilized sperm with 300 nM α-SNAP for 10 min at 37°C followed by 100 nM light chain of TeTx, and 0.5 mM CaCl2 for an additional 10 min (G, H, I). To evaluate whether preincubation with PTP1B ultimately leads to cis SNARE complex disassembly, we loaded sperm with this phosphatase (27 nM) and treated with TeTx without an exocytotic trigger (J, K, L). We then fixed and triple stained the cells with an anti-synaptobrevin2 antibody (“anti-syb 2”, red, left panels), FITC-PSA (to differentiate between reacted and intact sperm; green, central panels), and Hoechst 33342 (to visualize all cells in the field; blue, right panels). Asterisks in D, G and J indicate cells with intact acrosomes but without synaptobrevin2 immunostaining due to toxin cleavage. TeTx did not cleave synaptobrevin in resting sperm (A) but substantially decreased head labeling in sperm stimulated with calcium (D). Bars = 5 µm. M, percentage of cells showing synaptobrevin2 acrosomal staining from three independent experiments (mean ± S.E.M.). N, SLO-permeabilized spermatozoa were loaded with 27 nM PTP1B and incubated for 15 min at 37°C to disassemble cis SNARE complexes. Next, we added 300 nM α-SNAP and incubated for a further 15 min at 37°C. Finally, the AR was initiated by adding 0.5 mM CaCl2 and incubating for an additional 15 min (black bar). Controls (gray bars) include: background AR in the absence of any stimulation (control); AR stimulated by 0.5 mM CaCl2 (calcium); AR inhibited by 300 nM wild type α-SNAP (α-SNAP → calcium); and AR unperturbed by 27 nM PTP1B (PTP1B → calcium). Sperm were fixed and stained with FITC-PSA and the AR scored as described in the legend to Figure 1. Shown is the mean ± range of two independent experiments. O, we incubated SLO-permeabilized sperm with 300 nM wild type (top) or 50 nM M105I (bottom) α-SNAP for 10 min at 37°C followed by 0.5 mM CaCl2 for an additional 10 min. Subsequently, we added 100 nM light chain of TeTx and incubated for a further 10 min; we stopped toxin activity with 2.5 µM TPEN for 10 min. At the end of the incubation we released the α-SNAPs block with 300 nM NSF for 10 min at 37°C (black bars). To confirm that recombinant α-SNAP does not stimulate endogenous NSF to disassemble sperm cis SNARE complexes before adding an AR trigger (to dephosphorylate NSF), we modified the order of addition of reagents as indicated in the figure's labels and incubated as described above (open bars). We included the following controls (gray bars): background AR in the absence of any stimulation (control); AR stimulated by 0.5 mM CaCl2 (calcium); AR inhibited by 300 nM α-SNAP (α-SNAP → calcium) and 50 nM α-SNAP-M105I (α-SNAP-M105I → calcium); AR unperturbed by 300 nM NSF (NSF → calcium); AR inhibited by α-SNAPs and rescued by NSF (α-SNAPs → calcium → NSF); and AR inhibited by 100 nM TeTx (TeTx → calcium). Sperm were stained and the AR scored as described in the legend to Figure 1. Shown is the mean ± S.E.M. of at least three independent experiments. Actual percentages of reacted sperm for control and calcium ranged between 10–18 and 21–26% respectively.