SUMMARY
Type IV secretion systems (T4SS) are specialized protein complexes used by many bacterial pathogens for the delivery of effector molecules that subvert varied host cellular processes. Brucella spp. are facultative intracellular pathogens capable of survival and replication inside mammalian cells. Brucella T4SS (VirB) is essential to subvert lysosome fusion and to create an organelle permissive for replication. One possible role for VirB is to translocate effector proteins that modulate host cellular functions for the biogenesis of the replicative organelle. We hypothesized that proteins with eukaryotic domains or protein-protein interaction domains, among others, would be good candidates for modulation of host cell functions. To identify these candidates, we performed an in silico screen looking for proteins with distinctive features. Translocation of 84 potential substrates was assayed using adenylate cyclase reporter. By this approach, we identified six proteins that are delivered to the eukaryotic cytoplasm upon infection of macrophage-like cells and we could determine that four of them, encoded by genes BAB1_1043, BAB1_2005, BAB1_1275 and BAB2_0123, require a functional T4SS for their delivery. We confirmed VirB-mediated translocation of one of the substrates by immunofluorescence confocal microscopy, and we found that the N-terminal 25 amino acids are required for its delivery into cells.
INTRODUCTION
Type IV Secretion Systems (T4SS) are multiprotein complexes widespread in Archaea and Bacteria. These versatile secretion systems translocate DNA and protein substrates across the cell envelope generally by a contact-dependent mechanism (Alvarez-Martinez and Christie, 2009). A subset of these systems, present in Gram negative bacteria of medical importance, is specialized in the delivery of effector proteins directly into the cytosol of the target host cell to aid bacterial colonization and survival inside host tissues (Backert and Meyer, 2006; Christie et al., 2005). T4SS protein substrates have been shown to modulate varied cellular processes including apoptosis, vesicular traffic and ubiquitination (Franco et al., 2009; Ninio and Roy, 2007). As the number of type IV effector proteins continues to increase, it has been shown that proteins with eukaryotic-like domains or motifs are more likely to be effectors (Franco et al., 2009; de Felipe et al., 2008; Pan et al., 2008; Ninio and Roy, 2007; de Felipe et al., 2005; Cazalet et al., 2004; Nagai and Roy, 2003). However, most of them have not been functionally characterized and their biochemical activities and contribution to the intracellular lifestyle of bacterial pathogens remain unknown.
Brucellosis is a worldwide zoonosis caused by bacteria of the genus Brucella. The disease affects a wide variety of mammals and is transmitted to humans mainly by consumption of contaminated dairy products and by contact with infected animals (Godfroid et al., 2005). In animals, the disease primarily affects the reproductive system with concomitant loss in productivity of animals affected. Human brucellosis is a debilitating disease characterized by diverse pathological manifestations such as undulant fever, osteoarticular complications, endocarditis and several neurological disorders (Corbel, 1997; Nicoletti, 1989).
Brucella spp. are intracellular pathogens capable of infecting various cell types, including epithelial cells, placental trophoblasts, dendritic cells and macrophages (Gorvel, 2008). Once internalized, Brucella resides within the Brucella containing vacuole (BCV), a membrane-bound compartment where the bacterium survives and eventually proliferates. BCVs traffic along the endocytic pathway, interact with lysosomes and further mature into endoplasmic reticulum (ER) - derived replicative organelles (Starr et al., 2008; Celli et al., 2003; Pizarro-Cerda et al., 1998). Several studies have demonstrated that biogenesis of the ER-derived replicative niche is dependent on the functions of the Brucella T4SS (Sieira et al., 2004; Celli et al., 2003; Comerci et al., 2001; Delrue et al., 2001; Sieira et al., 2000; O’Callaghan et al., 1999).
Brucella T4SS (VirB), a major virulence determinant, has been shown to be essential for sustaining interactions and fusion events between BCVs and ER elements (Celli et al., 2003). Translocation of effector proteins into the host cell or the vacuolar membrane via VirB likely modulates host vesicular traffic, allowing the biogenesis of the ER-derived replicative organelle. Recently, two Brucella VirB substrates, VceA and VceC, were identified through the use of TEM1 β-lactamase fusion assays (de Jong et al., 2008). Both of these effectors are co-regulated with the virB genes. However, their biochemical activities and cellular targets during the infection process remain unknown.
Numerous putative type IV effector proteins have been identified via bioinformatic approaches (Chen et al., 2010; Ninio and Roy, 2007; de Felipe et al., 2005; Schulein et al., 2005). In Legionella pneumophila, systematic searches of genomes for eukaryotic-like genes have resulted in the identification of many confirmed and potential effector proteins (de Felipe et al., 2008; de Felipe et al., 2005; Cazalet et al., 2004; Chen et al., 2004; Nagai et al., 2002). In many cases, effector proteins with distinctive eukaryotic domains involved in protein-protein interactions, like ankyrin repeats and coiled coils motifs, have been shown to interfere with diverse host cellular processes to promote bacterial replication (Pan et al., 2008; Ninio and Roy, 2007).
In this work, we carried out an in silico approach to search all open reading frames of B. abortus S2308 genome for proteins with distinctive properties that would make them good candidates for modulation or evasion of host cell functions, a hallmark of T4SS substrates. After a bioinformatic analysis, 84 VirB substrate candidates were identified. Translocation of potential substrates into host cells was assayed using the Bordetella pertussis Adenylate Cyclase fusion approach (CyaA). We identified six proteins that are translocated to the host cell cytoplasm upon infection. Four of these proteins (BPE043, BPE005, BPE275 and BPE123) require a functional VirB system to be delivered into host target cells. VirB-dependent translocation of BPE123, was confirmed by confocal microscopy and we could also determine that the N-terminal 25 amino acids are required for VirB mediated delivery into host cells.
RESULTS
Identification of B. abortus putative effector proteins
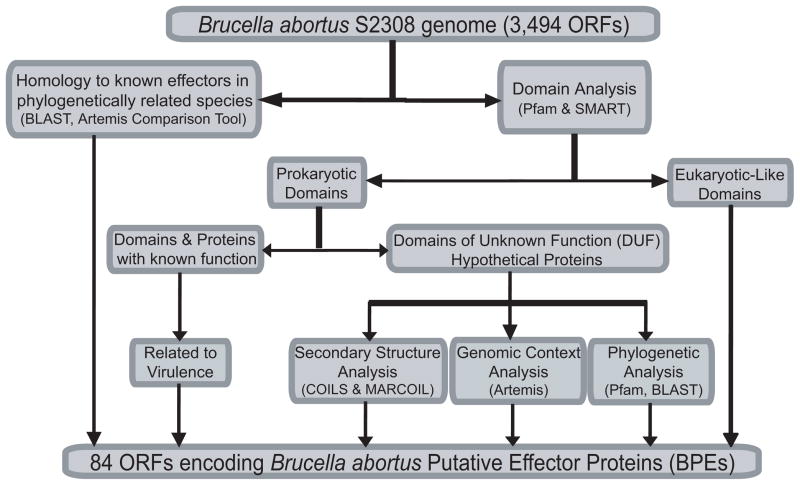
A bioinformatic genomewide screen was designed to identify B. abortus putative effector proteins based on the following criteria as depicted in Fig. 1.: i) homology to known effectors in related species; ii) the occurrence of eukaryotic-like domains or motifs; iii) proteins with domains known to be related to virulence; iv) proteins with unknown function but highly conserved in pathogens and symbionts from the α-proteobacteria division and v) proteins with distinctive features known to be involved in protein-protein interaction like coiled coils. Genomic context was also inspected and hypothetical proteins encoded by genes flanked by metabolic or housekeeping genes were discarded, while those flanked by other hypothetical genes, virulence related genes or next to a pathogenicity island were added to the list of putative effector proteins. The 3,494 annotated open reading frames (ORFs) of B. abortus strain 2308 (Chain et al., 2005) were scanned using the programs Pfam, SMART, COILS, MARCOIL, PSORTb, SignalP, TMHMM2.0, Artemis Comparison Tool (ACTv.6), Artemis v.10, and BLAST (Fig. 1). By this in silico procedure, 84 B. abortus putative effector proteins (BPEs) were identified (Table S3). The majority of the proteins are annotated as hypothetical proteins without predicted function. About 29% of the proteins are predicted to contain coiled-coils motifs and virulence related domains are present in 10% of the proteins. Eukaryotic-like domains, including patatin-like phospholipase, SH3-like domain and a GTP binding protein, among others, are present in about 23% of the proteins identified.
Figure 1.
Flow chart of the bioinformatic screening performed to identify B. abortus putative effector proteins (BPEs).
Translocation of candidate proteins into J774.A1 cells
To determine whether any of the ORFs listed in Table S3 encode proteins that are translocated into host cells upon infection, the B. pertussis calmodulin-dependent adenylate cyclase domain (CyaA) was fused to the C-terminus of BPEs. If the product of any of these genes is translocated to the host cell cytoplasm, delivery of the corresponding CyaA hybrid protein into J774.A1 cells would result in calmodulin-dependent activation of adenylate cyclase and enzymatic conversion of ATP to cAMP. Cytoplasmic cAMP concentration is subsequently determined by a quantitative ELISA assay. This approach was developed by Cornelis and colleagues to study the translocation of Yop effector proteins from Yersinia enterocolitica to host cells (Sory et al., 1995; Sory and Cornelis, 1994), and has been extensively used to identify proteins translocated by T4SS, such as L. pneumophila Dot/Icm and Bartonella henselae VirB systems (Chen et al., 2010; Pan et al., 2008; Schmid et al., 2006; Bardill et al., 2005; Nagai et al., 2005).
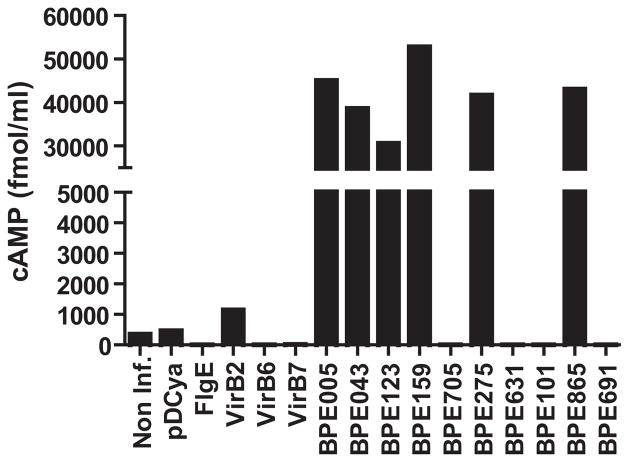
In the first screening round, 84 BPEs were fused to CyaA in pLFC vector under lac promoter, and expression of hybrid proteins in B. abortus was analyzed by Western Blot using anti-FLAG antibody (not shown). After a 5-hour infection, J774.A1 cells infected with B. abortus strains carrying CyaA fusions were lysed and cAMP levels were determined. As controls to detect non-specific delivery of CyaA hybrid proteins into host cells, well-known inner membrane, periplasmic and outer membrane proteins like VirB6, VirB7, VirB2 and FlgE (containing an N-terminal Sec secretion signal) were also included. After four independent replicates of the screening, we consistently observed that J774.A1 cells infected with B. abortus strains carrying CyaA fusions to BPE865, BPE005, BPE043, BPE159, BPE275, and BPE123 showed increased cAMP levels compared to the control strains, indicating that these six proteins are being translocated into host cells (Fig. S1).
In a second screening round, we selected the six positive candidates, four negative candidates and the negative controls from the previous screening for subcloning it as fusion to CyaA in pDCyaA vector under transcriptional control of B. abortus bcsp31 promoter, a strong and active promoter during the intracellular phase of B. abortus (Comerci et al., 1998). Expression of hybrid proteins in B. abortus was analyzed by Western Blot using anti-CyaA polyclonal antibodies (not shown). After a 5-hour infection, J774.A1 cells infected with B. abortus strains carrying CyaA fusions were lysed and cAMP levels were determined.
As shown in Fig. 2, J774.A1 cells infected with B. abortus strains carrying CyaA fusions to BPE865, BPE159, BPE275, BPE043, BPE005 and BPE123 showed a marked increase in cAMP levels compared to the control strains, indicating again that these proteins are being translocated into host cells. Importantly, these results demonstrate that 6 out of the 84 proteins identified using bioinformatic tools are delivered to the host cell cytoplasm upon infection. Notably, the use of the bcsp31 promoter increased the signal to noise ratio of the screening by an order of magnitude in comparison with the lac promoter. In consequence, we chose pDCyaA as expression vector for the rest of the study.
Figure 2. BPE translocation into J774.A1 cells.
Intracellular cAMP levels in J774.A1 cells infected with B. abortus strains expressing 10 BPE-CyaA hybrid proteins were measured after a 5 h infection. Controls included strains expressing VirB2-CyaA, VirB6-CyaA, VirB7-CyaA or FlgE-CyaA hybrid proteins as well as a wild-type strain containing no plasmid (WT) or a strain containing the CyaA domain alone (pDCyaA). Intracellular cAMP levels were also quantified in non-infected cells. cAMP levels are representative data from four independent experiments.
VirB dependent translocation of BPEs into J774.A1 cells
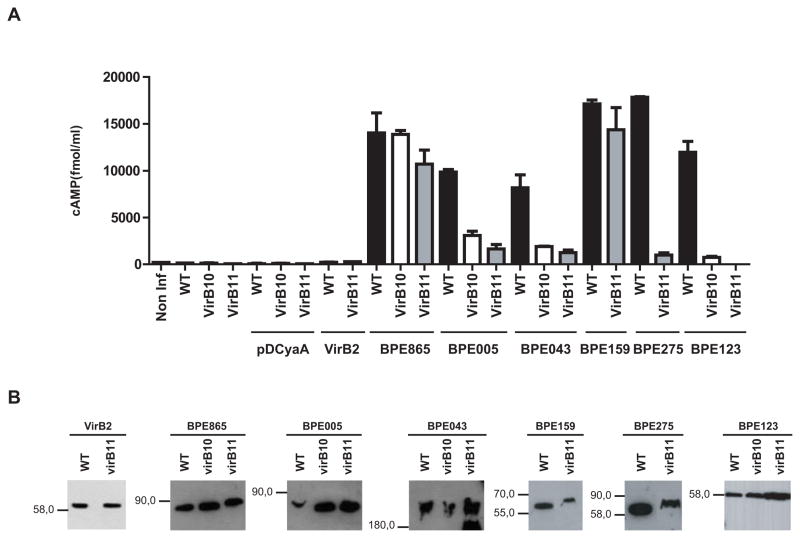
To determine if any of the six BPEs is translocated into host cells cytoplasm via Brucella VirB system, CyaA hybrid proteins in pDCyaA vector were expressed in virB mutant strains (virB10 and/or virB11), which lack a functional VirB system. At 5 h post-infection (p.i.), J774.A1 cells infected with the virB mutants expressing BPE865-CyaA and BPE159-CyaA did not show any significant decrease of cAMP levels compared to wild-type strain. In contrast, infection with virB mutants expressing BPE123-CyaA, BPE005-CyaA, BPE043-CyaA and BPE275-CyaA resulted in a significant decrease of intracellular cAMP concentration when compared to wild-type strain (Fig. 3A). The decrease in cAMP levels was not due to differential expression of the fusion proteins or intracellular killing of the virB mutants, since hybrid protein expression profiles assesed by western blot analysis as well as intracellular survival were equivalent between wild type and virB mutant strains (Fig. 3B and S2). These results demonstrate that BPE865 and BPE159 can enter the cytosol independently of the T4SS, indicating that Brucella proteins can be delivered through other pathways. More importantly, we show that BPE123, BPE005, BPE043 and BPE275 translocation requires the integrity of B. abortus T4SS, demonstrating that their delivery to the host cell is mediated by VirB system.
Figure 3. VirB-dependent translocation of BPEs into J774.A1 cells.
(A) Intracellular cAMP levels in J774.A1 cells infected with isogenic strains with a functional (WT) or nonfunctional VirB system (virB10 and virB11) expressing BPE-CyaA fusion proteins were measured after a 5 h infection. Controls included wild type strain (WT) and virB mutants (virB10 and virB11) either containing no plasmids, containing the CyaA domain alone (pDCyaA), or VirB2-CyaA fusion protein. Intracellular cAMP levels were also quantified in non-infected cells. Mean and SD are shown for one representative out of three independent experiments. (B) BPE-CyaA fusion protein levels of the indicated B. abortus strains were determined by immunoblot analysis with anti-CyaA antibodies.
BPE123 recruitment to the Brucella-containing vacuole is a VirB dependent process
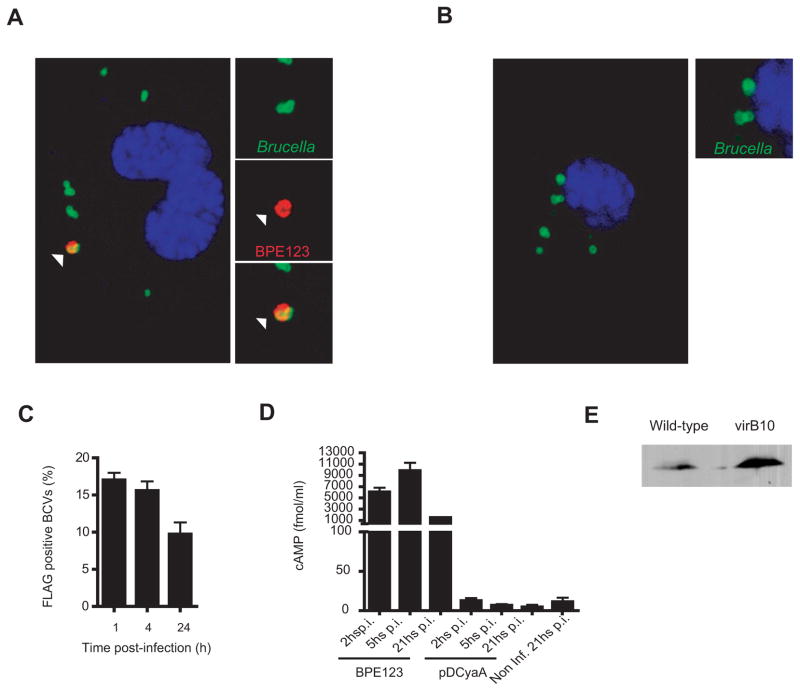
To independently address BPE123 translocation into host cells by VirB system, bone marrow-derived macrophages (BMDM) infected with wild-type and virB10 mutant encoding 3xFLAG-tagged BPE123 were analyzed by confocal microscopy with an anti-FLAG antibody. As the bacterium remains impermeable to the antibody, FLAG staining is indicative of protein translocation across the bacterial cell envelope. As shown in Fig. 4A, at 4 h p.i. BPE123 is localized to the proximity of wild-type BCVs. In contrast, BPE123 specific staining of BCVs was not observed in vacuoles containing virB10 mutants (Fig. 4B). In cells infected with wild type B. abortus encoding 3xFLAG-tagged BPE123, about 15% of BCVs were positive for FLAG at 1 and 4 h p.i., while this percentage decreased to 10% at 24 h p.i. (Fig 4C). Similarly, when analyzing intracellular cAMP levels during the time course of the infection with wild type B. abortus strain expressing CyaA fusion to BPE123, we found that cAMP concentration reaches a maximum between 2 and 5 h p.i. in J774.A1 cells (Fig. 4D), showing a translocation timing suitable for delivery across Brucella VirB system, which reaches a maximal activation at 5 h p.i. in J774.A1 cells (Sieira et al., 2004). Stability of FLAG-tagged BPE123 in both strains was assessed by immunoblot (Fig. 4E). These results are consistent with CyaA translocation experiments confirming that BPE123 requires a functional VirB system to be translocated into host cells where it remains associated to the BCVs membranes.
Figure 4. BPE123 recruitment to the Brucella containing vacuole (BCV) is a VirB dependent process.
Representative confocal micrographs of mouse BMDM infected with wild-type B. abortus (A) or a virB10 mutant strain (B) both expressing BPE123-3xFLAG (MOI 20:1). At 5 h p.i. cells were fixed and processed for immunostaining as described in materials and methods. Arrows indicate the location of BPE123 in the proximity of a wild-type BCV. (C) Percentage of FLAG positive BCVs scored during the time course of infection (mean± SD, n=3 independent experiments) (D) Intracellular cAMP levels in J774.A1 cells infected with B. abortus strain expressing BPE123-CyaA hybrid protein measured after 2, 5 and 21 h p.i. A strain containing the CyaA domain alone (pDCyaA) was included as a control. Intracellular cAMP levels were also quantified in non-infected cells at 21 h p.i. Mean and SD are shown for one representative out of three independent experiments. (E) BPE123-3xFLAG protein levels of the indicated B. abortus strains were determined by immunoblot analysis with anti-FLAG monoclonal antibody.
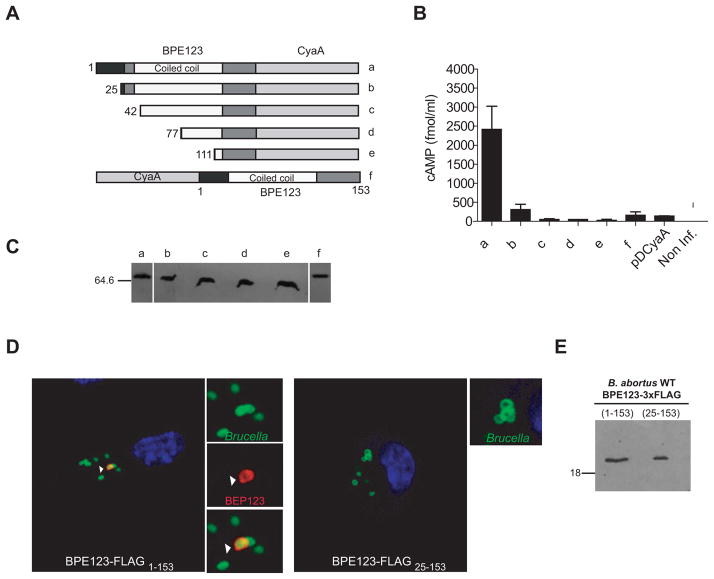
The N-terminal 25 amino acids of BPE123 are essential for translocation into host cells
In an attempt to determine the minimal fragment of BPE123 protein that would be sufficient for type IV translocation, truncated versions of BPE123-CyaA hybrid protein were generated by removing the putative signal peptide predicted for the first 25 amino acids, as well as different fragments of the central coiled coil sequence. We also constructed a fusion of CyaA to N-terminus of BPE123 (Fig. 5A). The strains carrying the plasmids encoding all these variants were used to infect J774.A1 macrophages and translocation of hybrid proteins into host cells was assessed by measuring the amount of intracellular cAMP at 5 h p.i. As shown in Fig. 5B, deletion of residues 1–25 in BPE123-CyaA hybrid protein, as well as fusion of CyaA to the N-terminus of BPE123, abolished BPE123 translocation into host cells. The decrease in cAMP levels was not due to differential expression or stability of the fusion proteins, since hybrid protein expression profiles assesed by immunoblot analysis were similar among all constructs (Fig. 5C). These results indicate that the N-terminal 25 amino acids, which are either absent in constructs b-e or fused to the C-terminus of CyaA in construct f (Fig. 5A), are critical for BPE123 translocation into host cells.
Figure 5. The N-terminal 25 amino acids of BPE123 are essential for translocation into host cells.
(A and B) J774.A1 cells were infected with B. abortus strains expressing full length and N-terminal truncations of BPE123-CyaA (a–e) as well as CyaA-BPE123 hybrid protein (f). Protein translocation was measured by determining the intracellular cAMP levels in J774.A1 cells infected for 5 h with B. abortus strains harboring the indicated plasmids. The wild type strain (2308) expressing the CyaA domain alone (pDCyaA) was included as a control. Intracellular cAMP was also quantified in non-infected cells. Mean and SD are shown for one representative out of three independent experiments. (C) Full length and N-terminal truncations of BPE123-CyaA (a–e) as well as CyaA-BPE123 (f) hybrid protein levels were determined by immunoblot analysis with anti-CyaA antibodies. (D) Representative confocal micrographs of mouse BMDM infected with wild-type B. abortus expressing full length (left panel) or truncated (right panel) BPE123-3xFLAG (MOI 20:1). At 5 h p.i. cells were fixed and processed for immunostaining as described in materials and methods. Arrows indicate the location of full length BPE123 in the proximity of a BCV. (D) Full length (lane 1) or truncated (lane 2) BPE123-3xFLAG protein levels of the indicated B. abortus strains were determined by immunoblot analysis with anti-FLAG monoclonal antibody.
Consistent with the N-terminal region of BPE123 being important for translocation, a FLAG tagged version of BPE123 bearing a deletion of residues 1–25 cannot be recruited to the proximities of BCVs, like full length BPE123 (Fig. 5D). Interestingly, when evaluating the stability of both FLAG tagged BPE123 proteins, we found that their electrophoretic mobilities were similar (Fig. 5E). This finding suggests that the full length protein with a putative secretion signal might be processed at the N-terminal cleavage site generating species with similar molecular weights. Altogether, these results indicate that the N-terminal 25-aa of BPE123 are necessary for its delivery into host cells by B. abortus VirB system.
BPE123 is not essential for B. abortus virulence
Previous studies have demonstrated that Brucella VirB system is essential to create an ER-derived organelle that supports Brucella replication inside host cells and for spleen colonization in experimentally infected mice (Celli et al., 2003; Comerci et al., 2001; Sieira et al., 2000; O’Callaghan et al., 1999). It is hypothesized that translocation of effector proteins via VirB modulates host vesicular traffic, allowing the biogenesis of the replicative organelle.
In order to assess the role of one of the identified VirB substrate in B. abortus virulence, we generated the mutant strain BPE123K and analyzed its behavior in intracellular replication and mice infection assays. As shown in Fig. S3A, similar numbers of wild-type and mutant CFUs were recovered during the course of the infection in J774.A1 macrophages, BMDM and in bone marrow-derived dendritic cells (BMDC). Consistent with these results, further characterization in HeLa cells revealed that the recruitment kinetics of the late endosome/lysosome glycoprotein LAMP-1 to BPE123K BCVs was indistinguishible from that of wild-type BCVs and that mutant BCVs further traffic to replicate in ER-derived compartments positive for calnexin like wild-type BCVs (Fig. S3B and S3C). Similarly, when the wild-type and the mutant strains were inoculated intraperitoneally in BALB/c mice, equal bacterial loads were obtained from spleens of infected mice at 30 and 60 days p.i. (Fig. S3D). Thus, a strain deficient in BPE123 has no measurable intracellular growth defect in host cells, indicating that this protein does not play an essential role in the establishment and maintenance of a vacuole that supports replication of B. abortus in cell culture conditions. Similarly, BPE123 is not required for spleen colonization in experimentally infected mice.
VirB substrates are highly conserved among Brucella species
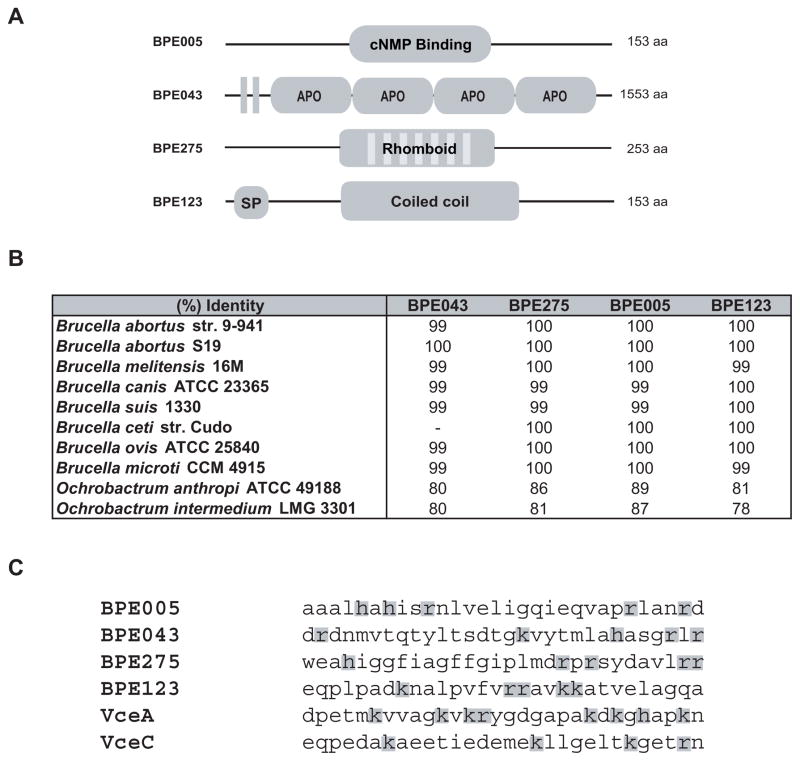
A schematic representation of the identified VirB substrates is shown in Fig 6A. BPE005, a protein of 153 amino acids bearing a cNMP binding domain, is annotated as a putative cyclic nucleotide-binding protein. These proteins may be regulatory subunits of cNMP dependent kinases, regulatory subunits of some other protein complex, or they might function to sequester cNMPs (McCue et al., 2000). BPE043 is a conserved hypothetical protein of 1553 amino acids, with prediction for two transmembrane domains and four apolipoprotein domains. Apolipoproteins participate in lipid transport as structural components of lipoprotein particles, cofactors for enzymes and ligands for cell-surface receptors. BPE275 contains 253 amino acids and is a member of rhomboid family, a ubiquitous family of serine proteases that function in varied cellular processes including intercellular signalling, parasitic invasion of host cells, and mitochondrial morphology (Kateete et al., 2010). BPE123 is a 17-kDa hypothetical protein with prediction for a signal peptide and a dimeric coiled coil motif. Coiled coils motifs are two or more α-helices forming a bundle structure usually involved in protein-protein interactions that participate in various cellular processes including membrane tethering and vesicle transport (Rose et al., 2005). Interestingly, relatively high coiled coil content is predicted for type III and type IV secretion systems substrates (Gazi et al., 2009). BPE005, BPE043 and BPE275 are proteins widespread in bacteria, whereas BPE123 only shows homology to prokaryotic proteins found in Ochrobactrum anthropi, O. intermedium and Bartonella bacilliformis. The four identified VirB substrates are highly conserved among Brucella species and close relatives including O. anthropi and O. intermedium (Fig 6B). Examination of C-terminal amino acid composition of the identified VirB substrates revealed a cluster of positive charged residues characteristic of some type IV substrates (Alvarez-Martinez and Christie, 2009) (Fig 6C). Although no obvious motif could be detected, this finding may indicate that a C-terminal domain might also be important for translocation of proteins across VirB system.
Figure 6. Amino acid sequence analysis of identified VirB substrates.
(A) Schematic representation of BPE proteins translocated across B. abortus VirB system. The number of amino acids is indicated on the right. cNMP: cyclic Nucleotide Mono-Phosphate; Vertical bars represent transmembrane domains; APO: apolipoprotein; SP: Sec Signal Peptide; (B) Percentage of identity between B. abortus 2308 VirB translocated BPEs and their orthologs in representative Brucella species and the phylogenetically related species O. anthropi and O. intermedium. (C) B. abortus VirB translocated substrates C-terminal 30 amino acids. Positively charged amino acids are shaded.
DISCUSSION
T4SS are membrane-associated protein complexes used by many Gram-negative pathogenic bacteria to translocate effector proteins that either hijack or interfere with host cell pathways. These effectors are delivered directly into host cells, with the exception of the B. pertussis Ptl system, which exports the A/B pertussis toxin to the extracellular milieu (Alvarez-Martinez and Christie, 2009). Brucella VirB system is one of the major virulence factors described so far, being essential for bacterial intracellular replication and colonization in experimentally infected mice (Celli et al., 2003; Comerci et al., 2001; Delrue et al., 2001; Sieira et al., 2000; O’Callaghan et al., 1999). Recently, two VirB substrates, VceC and VceA, were identified in a bioinformatic screen for genes co-regulated with virB genes (de Jong et al., 2008). However, their contribution to the intracellular lifestyle of Brucella remains to be uncovered.
In this report, we screened B. abortus 2308 genome for proteins with characteristics that would make them good candidates for translocation, such as eukaryotic-like domains, homology to known effectors in related species and structural features known to be involved in protein-protein interactions. We hypothesized that these proteins could be strong candidates for modulation of host cell functions during infection, making them potential substrates of Brucella VirB system. Here, we identified 84 B. abortus proteins (BPEs) with at least one of the mentioned properties, most of them annotated as hypothetical proteins without a predicted function. In contrast to what has been described for L. pneumophila, in which most of the 101 Dot/Icm effectors identified have distinctive eukaryotic domains (Franco et al., 2009), the occurrence of these kind of proteins in Brucella is relatively small (about 23% of the identified BPEs). Proteins with eukaryotic domains are assumed to have been acquired by interdomain horizontal gene transfer from eukaryotes to bacteria (Amor et al., 2005; de Felipe et al., 2005; Cazalet et al., 2004; Nagai et al., 2002). Thus, the large number of “eukaryotic-like” effector proteins in L. pneumophila could be explained by the fact that this bacterium, which occasionally infects human alveolar macrophages, is ubiquitously found in freshwater environments as an intracellular parasite of unicellular protozoans and in biofilms, whereas Brucella replication niche is essentially restricted to mammalian cells (Celli, 2006).
To determine whether any of these BPEs is translocated into host cells during infection, we decided to generate protein fusions to the N-terminus of the catalytic domain of the calmodulin-dependent adenylate cyclase of B. pertusiss, which provides a sensitive method for detecting protein translocation from bacteria to host cells (Chen et al., 2010; Ninio et al., 2009; Murata et al., 2006; Schmid et al., 2006; Nagai et al., 2005; Chen et al., 2004). All constructs were generated under the transcriptional control of a constitutive promoter in order to ensure intracellular expression of hybrid proteins. Although the CyaA fusion approach has failed to detect the translocation of aa 303–418 of Brucella VirB substrate VceC (de Jong et al., 2008), the use of this reporter has proved to be a sensitive method for detecting translocation of 6 BPEs into J774.A1 cells. It will be interesting to determine if the other BPEs are indeed translocated, either generating protein fusions to the C-terminus of CyaA or using other translocation reporters like TEM1 β–lactamase or CRAfT assay.
Importantly, we found that during infection of a macrophage-like cell line with strains expressing CyaA fusions to BPE123, BPE275, BPE043 and BPE005, intracellular cAMP levels were notably reduced when cells were infected with virB mutant strains. These results indicate that translocation of these proteins into J774.A1 cells is a VirB dependent process. In contrast, translocation of BPE865 and BPE159 did not require a functional T4SS, indicating that other translocation systems might function to deliver Brucella proteins across the phagosomal membrane.
Consistent with CyaA translocation experiments in J774.A1 cells, data from immunofluorescence microscopy indicate that upon VirB mediated translocation in BMDMs, BPE123 is localized to the phagosomal membrane surrounding the bacterium. Similarly, many L. pneumophila Dot/Icm substrates have been localized to the Legionella containing vacuole (Murata et al., 2006; Bardill et al., 2005; Luo and Isberg, 2004; Conover et al., 2003; Nagai et al., 2002). Restriction of BPE123 to the phagosomal membrane may be critical for concentration of its function to individual phagosomes, an important feature for controlling the biogenesis of the BCV. It should be noted that BPE123 was localized to the BCV membrane in afraction of intracellular bacteria raising the possibility that only bacteria that manage to translocate T4SS substrates become proficient for intracellular replication. Given that up to 90% of internalized brucellae are killed by the host cell (Kohler et al., 2003) it will be interesting to determine if the BCVs positive for BPE123 are committed to mature into ER-derived replicative organelles.
We showed thatthe 25-aa N-terminal region is essential for delivery of BPE123 into macrophage-like cells, as assessed by CyaA translocation experiments and immunofluorescence microscopy data. A Sec secretion signal is predicted for the N-terminal region of BPE123. These results raise the possibility that BPE123 is exported to the periplasm by the Sec translocon prior to its delivery into host cells by the VirB system. A two-step translocation process has already been demonstrated for pertussis toxin (Covacci and Rappuoli, 1993; Weiss et al., 1993) and VirD2, VirE2 and VirF, which interact with the periplasmic protein VirJ in A. tumefaciens (Pantoja et al., 2002). Another possible explanation for the N-terminal region of BPE123 being required for VirB translocation is that the transmembrane domain predicted for this region is necessary for protein insertion into the BCV. Regarding other Brucella VirB substrates, it has been recently demonstrated that the 20-aa C-terminal region of VceC is required for translocation of the fragment 303–418 into host cells, as determined by TEM1 reporter (de Jong et al., 2008). Thisfinding is consistent with previous studies indicating that a positively charged C-terminal region is the secretion signal for T4SS substrates (Cambronne and Roy, 2006). However, the C-terminal 20 aa of VceC are not conserved in Brucella species, indicating that a signal other than the C-terminal might be required for recognition and translocation of T4SS substrates. This is the case for Helicobacter pylori CagA protein, in which both N and C-terminal regions are important for translocation into host cells (Hohlfeld et al., 2006). In this respect, it should be mentioned that we also found a positively charged C-terminal regionin the four VirB substrates identified that might be relevant for translocation into host cells. In conclusion, although the presence of a C-terminal translocation signal has been demonstrated for many T4SS substrates, there might be specific requirements depending on the substrate and the T4SS studied. Further work aimedat deciphering the precise route of translocation may help clarify the nature and relevance of translocation signals.
BPE123 is a 153-aa protein highly conserved in all Brucella species sequenced. Homologous proteins are only found in O. anthropi, O. intermedium and B. bacilliformis. These species are phylogenetically related to Brucella and, like Brucella, arecapable of infecting eukaryotic cells (Berg et al., 2005; Dehio, 2005). A central coiled-coil motif is predicted for BPE123. Interestingly, these structural motifs mediating protein-protein interactions are widespread in substrates of type III and type IV secretion systems (Ninio and Roy, 2007; Delahay and Frankel, 2002). A strain lacking BPE123 showed no replication or traffic defects in cells and colonized mice spleens at wild-type rates. This finding is reminiscent of L. pneumophila, in which the lack of virulence defects is emerging as a general theme for Dot/Icm substrates (Machner and Isberg, 2006; Murata et al., 2006; Ninio et al., 2005; Nagai et al., 2002). Functional redundancy among VirB substrates is one possible explanation for the lack of a virulence phenotype in the mutant strain. This speculation could also explain the fact that genetic screens for attenuated mutants have failed to identify Brucella VirB substrates.
In silico screening combined with fusion to CyaA reporter led to the identification of four VirB substrates that may contribute to modulation or evasion of host cellular processes. At the moment, predicting the function of the identified Brucella T4SS substrates based solely on their domains is a difficult task. However, it is likely that the specific domains or motifs identified in these proteins are involved in protein-protein interactions with eukaryotic proteins that might be implicated in targeting varied cellular pathways. Functional characterization of these substrates will provide valuable information about the molecular mechanisms underlying pathogenesis of Brucella.
EXPERIMENTAL PROCEDURES
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Table S1. Brucella abortus strains were inoculated in tryptic soy agar (TSA) (Difco/Becton-Dickinson, Sparks, MD) or in tryptic soy broth (TSB) at 37°C on a rotary shaker for 16–20 h. When indicated, media were supplemented with 50μg/ml kanamycin, 5μg/ml nalidixic acid, 50μg/ml ampicillin and/or 3μg/ml gentamicin. All work with live B. abortus was performed in a biosafety level 3 laboratory facilityat University of San Martín. Escherichia coli strains were grown in Luria Broth (LB) at 37°C overnight. Antibiotics, when required, were added at the following concentrations: 50 μg/ml kanamycin and/or 100 μg/ml ampicillin.
DNA manipulations
Oligonucleotide primers used in this study are listed in Table S2. To generate plasmid pLFC, bcsp31 ribosome binding site was excised from pDCyaA (see below) using HindIII-SmaI restriction sites and the fragment was ligated to the HindIII-SmaI sites of pBBR1-MCS4 downstream of the lac promoter (Kovach et al., 1995). The resulting plasmid was digested with SmaI-SacI restriction enzymes and a DNA fragment coding for Bordetella pertussis adenylate cyclase catalytic domain (CyaA), amplified from pMS107 (Sory and Cornelis, 1994) using primers START CyaA and STOP CyaA, was ligated into the corresponding sites. A fragment coding for 3xFLAG epitope was amplified from pBAD24-3xFLAG (Spano et al., 2008) using primers 3FLAG·SpeI and 3FLAG XbaI, and was ligated into the SpeI site of the vector to generate pLFC plasmid.
To generate plasmid pDCyaA, bcsp31 promoter, including the ribosome binding site, was amplified from B. abortus S2308 genomic DNA using primers BCSP31 EcoRI and BCSP31 SmaI, and the PCR product with flanking EcoRI-SmaIsites was ligated into the EcoRI-SmaIsites of pDK51 (Marchesini et al., 2004)to drive expression of CyaA fusion proteins. In the resulting plasmid, a XbaI-SacI DNA fragment coding for CyaA, generated by PCR amplification from plasmid pMS107 using primers CyaA XbaI and CyaA SacI, was ligated into the corresponding sites to generate plasmid pDCyaA. To generate plasmids coding for fusions to the N-terminus of CyaA, BamHI/SpeI DNA fragments coding for B. abortus candidate proteins were obtained by PCR amplification with primers carrying BamHI/SpeI sites and ligated into the corresponding sites of pDCyaA or pLFC plasmids. Oligonucleotide sequences used for amplification of the selected ORFs are listed in Table S2. To generate the plasmid coding for CyaA-BPE123, a DNA fragment coding for CyaA was amplified using primers CyaA BamHI and CyaA SpeI while the gene coding for BPE123 was amplified using primers 20123 XbaI and 20123 SacII. Both DNA fragments were ligated in the corresponding sites of pDK51 under bcsp31 gene promoter. To generate BPE123 N-terminal deletions, oligonucleotides 123 BamHI-25, 123 BamHI-42, 123 BamHI-77 and 123 BamHI-111 were used, where the numbers indicate the first codon of bpe123 to be amplified. Plasmids expressing CyaA fusion proteins were introduced in B. abortus strains by biparental mating.
For constructing vectors expressing C-terminal 3xFLAG-tagged BPE123 proteins, the plasmid pBAD24-3xFLAG was used. DNA fragments coding for full length (aa 1–153) or truncated (aa 25–153) BPE123 were amplified by PCR using primers 20123 BamHI/20123 NcoI or 123 BamHI-25/20123 NcoI, respectively. The PCR products were inserted by the flanking BamHI/NcoI sites in the corresponding sites of pBAD24-3xFLAG to generate in frame fusions to 3xFLAG epitope. Then, BamHI/XbaI fragments encoding protein fusions were ligated in the corresponding sites of pBBR1 MCS-4 to generate pBPE123-FLAG (1-153) and pBPE123-FLAG (25-153). Plasmids expressing 3xFLAG tagged fusion proteins were introduced in B. abortus strains by biparental mating. The integrity of all constructs was confirmed by sequence analysis.
Bacterial infection and replication assays
Cell lines were maintained and plated as previously described (Bukata et al., 2008). To obtain bone marrow-derived macrophages (BMDMs), bone marrow cells were isolated from femurs of 6 to 10-week-old C57BL/6 female mice and differentiated into macrophages as described (Celli et al., 2005). Bone marrow-derived dentritic cells (BMDCs) were prepared from 7–8 week-old female C57BL/6 mice as described (Salcedo et al., 2008). Cells (5 ×104/well) were seeded on 24-well plates in media without antibiotics 24 h before infection. B. abortus infections were carried out at the indicated multiplicity of infection (MOI). Bacteria were centrifuged onto cells at 400 × g for 10 min. After 30 min (J774.A1 cells, BMDMs and BMDCs) or 60 min (HeLa cells) wells were gently washed three times with phosphate-buffered saline (PBS) and incubated for 60 min with fresh medium containing 50 μg/ml gentamicin and 100 μg/ml streptomycin to kill noninternalized bacteria. Thereafter, antibiotics concentrations were decreased to 10 μg/ml gentamicin and 20 μg/ml streptomycin. At the indicated times, infected cells were either washed three times with PBS and lysed with 500 μl 0.1% Triton X-100 in H2O (Sigma-Aldrich) or processed for immunoflourescence staining as described below. The intracellular CFU counts were determined by plating serial dilutions on TSA with the appropriated antibiotic.
CyaA translocation assay
Translocation of potential substrates into host cells was assayed using the CyaA fusion approach. After infection of J774.A1 cells (MOI 250:1) for the indicated times in 96-wells plates (105 cells/well), cells were gently washed five times with PBS and lysed. Intracellular cAMP levels were determined by Direct cAMP Enzyme Immunoassay Kit (Sigma, CA200) as described by the manufacturer.
Immunoblot analysis
To monitor the level of CyaA or 3xFLAG fusion proteins, B. abortus strains were grown in TSB and harvested at stationary phase. Equivalent bacterial pellets were resuspended in Laemmli buffer and samples were subjected to SDS-PAGE. Proteins were transferred onto nitrocellulose membranes using semi-dry transfer. Immunobloting was performed using polyclonal anti-CyaA or monoclonal anti-FLAG M2 (Sigma-Aldrich) antibodies. A recombinant histidine-tagged CyaA domain (aa 2–405) was used to prepare a mouse serum against CyaA by using a standard scheme of immunization.
Immunofluorescence microscopy
Eukaryotic cells were plated on glass coverslips and infected as described above. At the indicated times the coverslips were washed with PBS and the cells were fixed for 15 min in 3% paraformaldehyde (pH 7.4) at 37°C. Coverslips were then processed for immunofluorescence labeling as previously described (Comerci et al., 2001), except for anti-FLAG M2, that was diluted in 0.2 % Triton X-100 instead of 0.1% saponin for cell permeabilization. After immunofluorescence labeling, the coverslips were mounted onto slides with FluorSave (Calbiochem). Samples were either examined on a Nikon microscope (Eclipse E600) or a Zeiss LSM 510 laser scanning confocal microscope for image acquisition. Images of 1024 × 1024 pixels were then assembled using Adobe Photoshop CS.
Antibodies and reagents
The primary antibodies used for immunofluorescence microscopy were cow anti-Brucella polyclonal antibody, mouse anti-FLAG M2 (Sigma-Aldrich), mouse anti-human LAMP-1 H4A3 (Developmental Studies Hybridoma Bank, National Institute of Child Health and Human Development, University of Iowa) and rabbit polyclonal anti-calnexin (Stressgen). The secondary antibodies used were FITC-conjugated donkey anti-cow IgG, Texas Red-conjugated donkey anti-mouse or anti-rabbit IgG (Jackson ImmunoResearch). For DNA staining, Hoechst dye at 2 μg/ml (final concentration) or To-Pro3 (Invitrogen) were used.
Generation of BPE123K mutant
A DNA fragment of 500 bp coding for BPE123 was amplified by PCR using primers 20123 BamHIand 20123 SpeI. The PCR product was ligated to pGem-T-Easy (Promega) and the resulting plasmid was linearized with HindIII and blunt ended with T4 DNA polymerase (New England Biolabs). Linearized pGem-T-bpe123 was ligated to a HincIIDNA fragment coding for a nonpolar kanamycin resistance cassette to generate pGem-T-bpe123::Kan. This plasmid was electroporated into B. abortus 2308 where it is incapable of autonomous replication. Homologous recombination events were selected using kanamycin resistance and ampicillin sensitivity. PCR analyses showed that the bpe123 wild-type gene was replaced by the disrupted one. The mutant strain obtained was called BPE123K.
Mice infection
Eight-week-old female BALB/c mice were intraperitoneally inoculated with 105 CFU of B. abortus strains in PBS. At the indicated times post-infection, spleens from infected mice were removed and homogenized in 2 ml of PBS. Tissue homogenates were serially diluted and plated in duplicate on TSA with the appropriate antibiotic. CFU were counted after 3 to 4 days of incubation at 37°C. Infected mice were kept in cages within a biosafety level 3 facility.
URLs
PSORTb v.2.0.4 (Gardy et al., 2005) is available at http://www.psort.org/psortb/; SignalP 3.0 (Bendtsen et al., 2004) at http://www.cbs.dtu.dk/services/SignalP/; COILS (Lupas et al., 1991) at http://www.ch.embnet.org/software/COILS_form.html; Multicoil (Wolf et al., 1997) at http://groups.csail.mit.edu/cb/multicoil/cgi-bin/multicoil.cgi; SMART (Letunic et al., 2009; Schultz et al., 1998) at http://smart.embl-heidelberg.de/; Pfam 23.0 (Finn et al., 2008) at http://pfam.janelia.org/; MARCOIL (Delorenzi and Speed, 2002) at http://www.isrec.isb-sib.ch/webmarcoil/webmarcoilC1.html; TMHMM2.0 (Krogh et al., 2001) at http://www.cbs.dtu.dk/services/TMHMM/.
Supplementary Material
Acknowledgments
We would specially like to dedicate this work to the memory of Dr. Rodolfo A. Ugalde.
We are also grateful to Dr. Juan E. Ugalde for supplying pBAD24-3xFLAG. This study was supported by a grant from the National Institutes of Health (grant NIAID-NIH-RO1AI078891-01), grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT projects PICT2005 38272 & PICT2006 651) and an EMBO Short-Term Fellowship (ASTF 21-2007) to MIM. http://www.conicet.gov.ar/; http://www.agencia.mincyt.gov.ar/; http://www.nih.gov/; http://www.embo.org. DJC is member of the Scientific Research Career from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET); MIM is a postdoctoral fellow from CONICET; CKH is a PhD fellow from ANPCyT. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor JC, Swails J, Zhu X, Roy CR, Nagai H, Ingmundson A, et al. The structure of RalF, an ADP-ribosylation factor guanine nucleotide exchange factor from Legionella pneumophila, reveals the presence of a cap over the active site. J Biol Chem. 2005;280:1392–1400. doi: 10.1074/jbc.M410820200. [DOI] [PubMed] [Google Scholar]
- Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol. 2006;9:207–217. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Bardill JP, Miller JL, Vogel JP. IcmS-dependent translocation of SdeA into macrophages by the Legionella pneumophila type IV secretion system. Mol Microbiol. 2005;56:90–103. doi: 10.1111/j.1365-2958.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol. 2005;7:1673–1685. doi: 10.1111/j.1462-2920.2005.00891.x. [DOI] [PubMed] [Google Scholar]
- Bukata L, Altabe S, de Mendoza D, Ugalde RA, Comerci DJ. Phosphatidylethanolamine synthesis is required for optimal virulence of Brucella abortus. J Bacteriol. 2008;190:8197–8203. doi: 10.1128/JB.01069-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne ED, Roy CR. Recognition and delivery of effector proteins into eukaryotic cells by bacterial secretion systems. Traffic. 2006;7:929–939. doi: 10.1111/j.1600-0854.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- Cazalet C, Rusniok C, Bruggemann H, Zidane N, Magnier A, Ma L, et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nat Genet. 2004;36:1165–1173. doi: 10.1038/ng1447. [DOI] [PubMed] [Google Scholar]
- Celli J. Surviving inside a macrophage: the many ways of Brucella. Res Microbiol. 2006;157:93–98. doi: 10.1016/j.resmic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Celli J, Salcedo SP, Gorvel JP. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc Natl Acad Sci U S A. 2005;102:1673–1678. doi: 10.1073/pnas.0406873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerci DJ, Pollevick GD, Vigliocco AM, Frasch AC, Ugalde RA. Vector development for the expression of foreign proteins in the vaccine strain Brucella abortus S19. Infect Immun. 1998;66:3862–3866. doi: 10.1128/iai.66.8.3862-3866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerci DJ, Martinez-Lorenzo MJ, Sieira R, Gorvel JP, Ugalde RA. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- Conover GM, Derre I, Vogel JP, Isberg RR. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol Microbiol. 2003;48:305–321. doi: 10.1046/j.1365-2958.2003.03400.x. [DOI] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A, Rappuoli R. Pertussis toxin export requires accessory genes located downstream from the pertussis toxin operon. Mol Microbiol. 1993;8:429–434. doi: 10.1111/j.1365-2958.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Chain PS, Comerci DJ, Tolmasky ME, Larimer FW, Malfatti SA, Vergez LM, et al. Whole-genome analyses of speciation events in pathogenic Brucellae. Infect Immun. 2005;73:8353–8361. doi: 10.1128/IAI.73.12.8353-8361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Banga S, Mertens K, Weber MM, Gorbaslieva I, Tan Y, et al. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1010485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, de Felipe KS, Clarke M, Lu H, Anderson OR, Segal G, Shuman HA. Legionella effectors that promote nonlytic release from protozoa. Science. 2004;303:1358–1361. doi: 10.1126/science.1094226. [DOI] [PubMed] [Google Scholar]
- Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu Rev Microbiol. 2005;59:451–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe KS, Pampou S, Jovanovic OS, Pericone CD, Ye SF, Kalachikov S, Shuman HA. Evidence for acquisition of Legionella type IV secretion substrates via interdomain horizontal gene transfer. J Bacteriol. 2005;187:7716–7726. doi: 10.1128/JB.187.22.7716-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog. 2008;4:e1000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MF, Sun YH, den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol Microbiol. 2008;70:1378–1396. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C. Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol. 2005;3:621–631. doi: 10.1038/nrmicro1209. [DOI] [PubMed] [Google Scholar]
- Delahay RM, Frankel G. Coiled-coil proteins associated with type III secretion systems: a versatile domain revisited. Mol Microbiol. 2002;45:905–916. doi: 10.1046/j.1365-2958.2002.03083.x. [DOI] [PubMed] [Google Scholar]
- Delorenzi M, Speed T. An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics. 2002;18:617–625. doi: 10.1093/bioinformatics/18.4.617. [DOI] [PubMed] [Google Scholar]
- Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, et al. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, et al. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco IS, Shuman HA, Charpentier X. The perplexing functions and surprising origins of Legionella pneumophila type IV secretion effectors. Cell Microbiol. 2009;11:1435–1443. doi: 10.1111/j.1462-5822.2009.01351.x. [DOI] [PubMed] [Google Scholar]
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M, Brinkman FS. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21:617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- Gazi AD, Charova SN, Panopoulos NJ, Kokkinidis M. Coiled-coils in type III secretion systems: structural flexibility, disorder and biological implications. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01297.x. [DOI] [PubMed] [Google Scholar]
- Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, et al. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res. 2005;36:313–326. doi: 10.1051/vetres:2005003. [DOI] [PubMed] [Google Scholar]
- Gorvel JP. Brucella: a Mr “Hide” converted into Dr Jekyll. Microbes Infect. 2008;10:1010–1013. doi: 10.1016/j.micinf.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld S, Pattis I, Puls J, Plano GV, Haas R, Fischer W. A C-terminal translocation signal is necessary, but not sufficient for type IV secretion of the Helicobacter pylori CagA protein. Mol Microbiol. 2006;59:1624–1637. doi: 10.1111/j.1365-2958.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Kateete DP, Okee M, Katabazi FA, Okeng A, Asiimwe J, Boom HW, et al. Rhomboid homologs in mycobacteria: insights from phylogeny and genomic analysis. BMC Microbiol. 2010;10:272. doi: 10.1186/1471-2180-10-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Michaux-Charachon S, Porte F, Ramuz M, Liautard JP. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 2003;11:215–219. doi: 10.1016/s0966-842x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, 2nd, Peterson KM. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART 6: recent updates and new developments. Nucleic Acids Res. 2009;37:D229–232. doi: 10.1093/nar/gkn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZQ, Isberg RR. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc Natl Acad Sci U S A. 2004;101:841–846. doi: 10.1073/pnas.0304916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Marchesini MI, Ugalde JE, Czibener C, Comerci DJ, Ugalde RA. N-terminal-capturing screening system for the isolation of Brucella abortus genes encoding surface exposed and secreted proteins. Microb Pathog. 2004;37:95–105. doi: 10.1016/j.micpath.2004.06.001. [DOI] [PubMed] [Google Scholar]
- McCue LA, McDonough KA, Lawrence CE. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 2000;10:204–219. doi: 10.1101/gr.10.2.204. [DOI] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–977. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- Nagai H, Roy CR. Show me the substrates: modulation of host cell function by type IV secretion systems. Cell Microbiol. 2003;5:373–383. doi: 10.1046/j.1462-5822.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- Nagai H, Cambronne ED, Kagan JC, Amor JC, Kahn RA, Roy CR. A C-terminal translocation signal required for Dot/Icm-dependent delivery of the Legionella RalF protein to host cells. Proc Natl Acad Sci U S A. 2005;102:826–831. doi: 10.1073/pnas.0406239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti P. Brucellosis: clinical and laboratory aspects. In: Corbel EJYaMJ., editor. Relantionship between animal and human disease. Boca Raton, Florida: CRC Press, Inc; 1989. pp. 41–51. [Google Scholar]
- Ninio S, Roy CR. Effector proteins translocated by Legionella pneumophila: strength in numbers. Trends Microbiol. 2007;15:372–380. doi: 10.1016/j.tim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Ninio S, Celli J, Roy CR. A Legionella pneumophila effector protein encoded in a region of genomic plasticity binds to Dot/Icm-modified vacuoles. PLoS Pathog. 2009;5:e1000278. doi: 10.1371/journal.ppat.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio S, Zuckman-Cholon DM, Cambronne ED, Roy CR. The Legionella IcmS-IcmW protein complex is important for Dot/Icm-mediated protein translocation. Mol Microbiol. 2005;55:912–926. doi: 10.1111/j.1365-2958.2004.04435.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, et al. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- Pan X, Luhrmann A, Satoh A, Laskowski-Arce MA, Roy CR. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science. 2008;320:1651–1654. doi: 10.1126/science.1158160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja M, Chen L, Chen Y, Nester EW. Agrobacterium type IV secretion is a two-step process in which export substrates associate with the virulence protein VirJ in the periplasm. Mol Microbiol. 2002;45:1325–1335. doi: 10.1046/j.1365-2958.2002.03098.x. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, et al. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A, Schraegle SJ, Stahlberg EA, Meier I. Coiled-coil protein composition of 22 proteomes--differences and common themes in subcellular infrastructure and traffic control. BMC Evol Biol. 2005;5:66. doi: 10.1186/1471-2148-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MC, Scheidegger F, Dehio M, Balmelle-Devaux N, Schulein R, Guye P, et al. A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog. 2006;2:e115. doi: 10.1371/journal.ppat.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulein R, Guye P, Rhomberg TA, Schmid MC, Schroder G, Vergunst AC, et al. A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci U S A. 2005;102:856–861. doi: 10.1073/pnas.0406796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A. 1998;95:5857–5864. doi: 10.1073/pnas.95.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieira R, Comerci DJ, Sanchez DO, Ugalde RA. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J Bacteriol. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieira R, Comerci DJ, Pietrasanta LI, Ugalde RA. Integration host factor is involved in transcriptional regulation of the Brucella abortus virB operon. Mol Microbiol. 2004;54:808–822. doi: 10.1111/j.1365-2958.2004.04316.x. [DOI] [PubMed] [Google Scholar]
- Sory MP, Cornelis GR. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- Sory MP, Boland A, Lambermont I, Cornelis GR. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci U S A. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano S, Ugalde JE, Galan JE. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe. 2008;3:30–38. doi: 10.1016/j.chom.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- Weiss AA, Johnson FD, Burns DL. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci U S A. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf E, Kim PS, Berger B. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 1997;6:1179–1189. doi: 10.1002/pro.5560060606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock DM, Crowther PJ, Doherty J, Jefferson S, DeCruz E, Noyer-Weidner M, et al. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–3478. doi: 10.1093/nar/17.9.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.