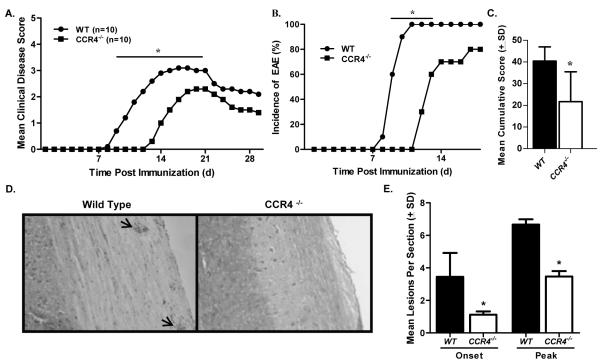
Figure 2. Disease is delayed in CCR4−/− mice.
WT and CCR4−/− mice were immunized with MOG35-55 emulsified in CFA and monitored for clinical disease development. (A) CCR4−/− mice showed a significant decrease in clinical disease as compared to WT mice at early time points (days 9-17). The data is expressed as daily mean clinical disease score post immunization. N=10 mice per group. (B) Disease incidence was significantly diminished in CCR4−/− mice early in disease. (C) Mean cumulative disease score (±SD) was calculated for each group and determined to be significantly reduced in CCR4−/− mice. (D) Knockout mice showed reduced histological disease as determined by microscopic analysis of H&E stained spinal cord sections at onset and peak of clinical disease. Representative photomicrographs (original magnification, 100×) of spinal cord sections from WT and CCR4−/− mice at onset of clinical disease. Arrows indicate mononuclear cell infiltrate. (E) Quantitative analysis of histological disease was performed by calculating the mean number (±SD) of inflammatory foci per section at onset and peak of disease. These results are representative of three separate experiments. *, p<0.05.