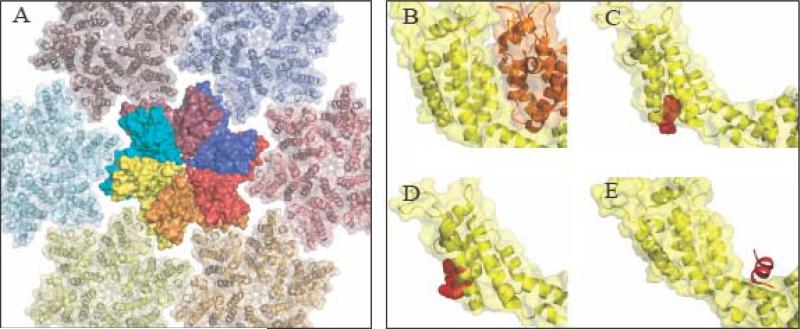
Figure 1.
Intersubunit Interactions and Inhibitor Binding Sites in HIV-1 Capsid Protein. (A) The structure of the mature CA hexamer. One full hexamer is shown in space filling form, and six surrounding hexamers are shown as ribbon diagrams. The individual subunits of the full hexamer are displayed with different colors. Note how the C-terminal domain of one subunit sits underneath the N-terminal domain of the adjacent (counter-clockwise) subunit. (B) Structure of two interacting CA subunits extracted from the mature HIV-1 hexamer. The NTD of both subunits is pictured. The CTD of the yellow subunit contacts the NTD of the adjacent (orange) subunit. This NTD/CTD interaction is unique to the mature form. (C) CAP-1 (red) is a small organic compound that binds to the NTD of HIV-1 CA and prevents assembly of the mature lattice, presumably by interfering with the NTD/CTD interaction. (D). (D) PF-74 (red) is a small organic compound that binds to the NTD of HIV-1 CA and destabilizes cores. (E) CAP-1 (red) is a small α-helical peptide that binds to the CA CTD and prevents assembly of both the immature and mature forms. The compounds were docked into subunits extracted from the hexamer crystal structure (PDB code 3MGE) by alignment of overlapping regions using domain/compound co-crystal structures (PDB Code 2JPR for CAP-1, 2XDE for PF-74, and 2BU0 for CAI) and hence do not reflect conformational changes induced by compound docking. The viewpoint varies slightly from panel to panel to best illustrate the relevant interaction.