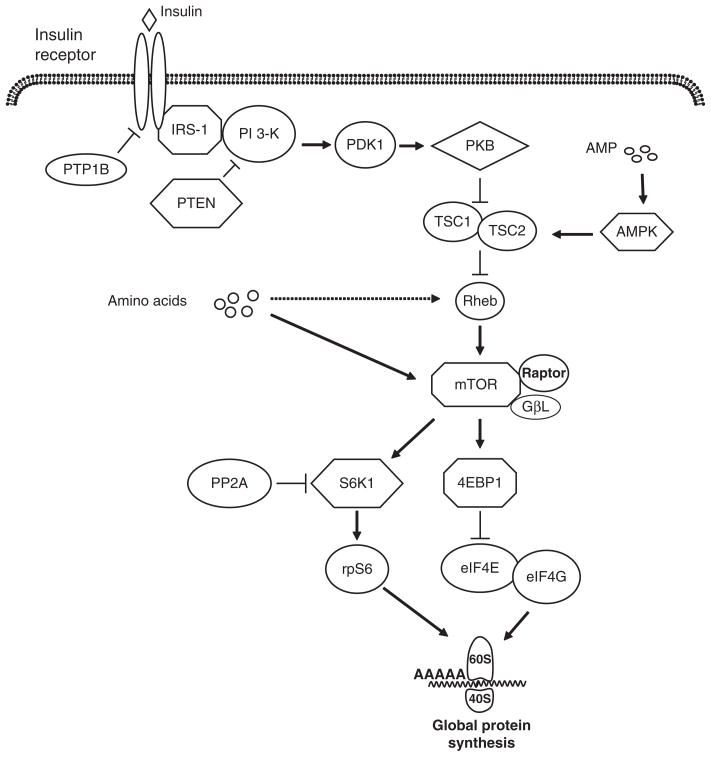
Figure 1.
Insulin and amino acid signaling pathways that lead to the stimulation of translation initiation. The binding of insulin to its receptor activates the insulin receptor and insulin receptor substrate-1 (IRS-1), followed by the activation of phosphoinositide-3 kinase (PI 3-kinase). Activated PI3 kinase stimulates phosphoinositide-dependent kinase 1 (PDK-1) and protein kinase B (PKB) activation. Phosphorylation of PKB inactivates tuberous sclerosis complex 1 and 2 (TSC1/2), inducing the activation of Rheb and mammalian target of rapamycin (mTOR). Both amino acids and insulin activate mTOR, which exists in a complex with raptor and G protein β-subunit-like protein (GβL). Activated mTOR phosphorylates ribosomal protein S6 kinase 1 (S6K1) and eukaryotic initiation factor (eIF) 4E-binding protein-1 (4EBP1). Phosphorylation of S6K1 enhances the activation of ribosomal subunit S6 (rpS6), which increases the translation of specific mRNA. Phosphorylated 4EBP1 releases eIF4E from an inactive complex with 4EBP1, enabling the formation of the active eIF4E-eIF4G complex that mediates mRNA binding to the ribosome. Insulin signaling can be attenuated by a number of signaling proteins. These include protein tyrosine phosphatase-1B (PTP-1B), which dephosphorylates the insulin receptor and IRS-1; phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which inactivates PI 3-kinase; and protein phosphatase 2A (PP2A), which decreases activation of PKB and S6K1. An increase in adenosine monophosphate (AMP) levels enhances AMP kinase activation, increasing TSC1/2 complex activation and decreasing mTOR activation.