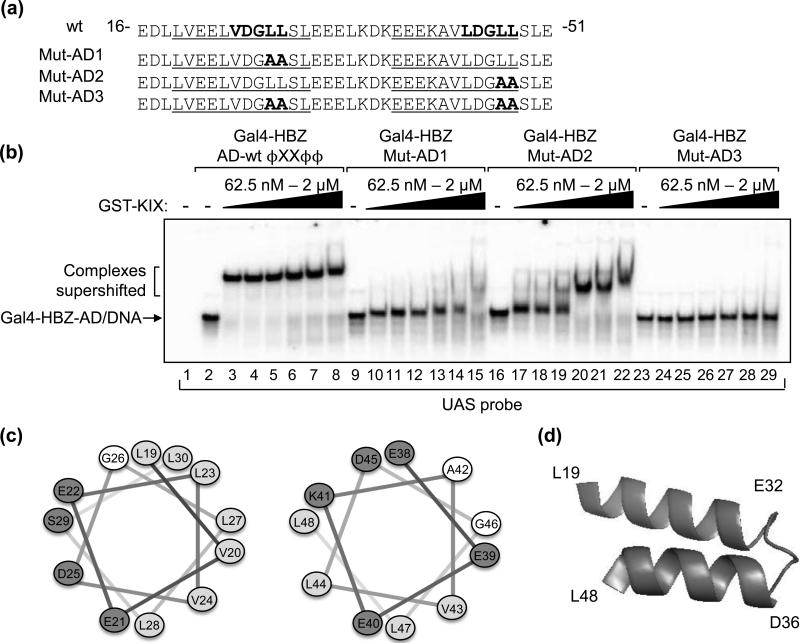
Figure 2. Two ΦXXΦΦ motifs within predicted amphipathic α-helices of HBZ-AD mediate binding to KIX.
(a) Amino acids 16 to 51 of HBZ are shown. Indicated in bold are the two ΦXXΦΦ motifs in the wild-type (wt) sequence and amino acid substitutions in HBZ-Mut-AD1, HBZ-Mut-AD2 and HBZ-Mut-AD3. Sequences predicted to form amphipathic α-helices are underlined. (b) Each ΦXXΦΦ motif contributes to the affinity of Gal4-HBZ-AD for GST-KIX. EMSA reactions contained UAS probe (3.25 nM) and 1nM Gal4-HBZ-AD wt, Gal4-HBZ-Mut-AD1, Gal4-HBZ-Mut-AD2 or Gal4-HBZ-Mut-AD3 as indicated. GST-KIX was titrated into reactions (62.5 nM, 125 nM, 250 nM, 500 nM, 1 μM, and 2 μM) as indicated. (c) Helical wheels show uncharged, hydrophobic, and charged amino acids as unshaded, lightly shaded and darkly shaded, respectively. (d) The schematic shows that the two amphipathic α-helices in HBZ-AD are predicted to form a hairpin structure.