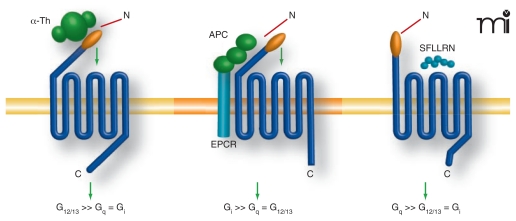
Figure 1. Proteases and peptide agonists display biased agonism at protease-activated receptor 1 (PAR1).
PAR1 is a seven-transmembrane G protein–coupled receptor activated by a unique proteolytic mechanism. Thrombin (α-Th), a serine protease, binds to and proteolytically cleaves the N terminus of PAR1, unmasking a new N terminal domain that then acts as a tethered ligand, binding intramolecularly to the body of the receptor to trigger transmembrane signaling. Thrombin-activated PAR1 preferentially couples to G12/13 proteins, which signal to RhoGEFs and result in RhoA activation and endothelial permeability. Synthetic peptide agonists that mimic the newly exposed N terminus–tethered ligand domain, SFLLRN, can activate PAR1 independently of proteolysis. Activation of PAR1 by addition of agonist peptides, SFLLRN or TFLLRNPNDK, promotes preferential coupling to Gq over G12/13. Activated protein C (APC), an anti-coagulant serine protease, requires endothelial protein C receptor (EPCR) as a co-factor and cleaves and activates PAR1. However, in contrast to thrombin, activation of PAR1 by APC promotes endothelial barrier protective signaling predominantly through Gi coupling and is dependent on caveolae, a subtype of lipid rafts.