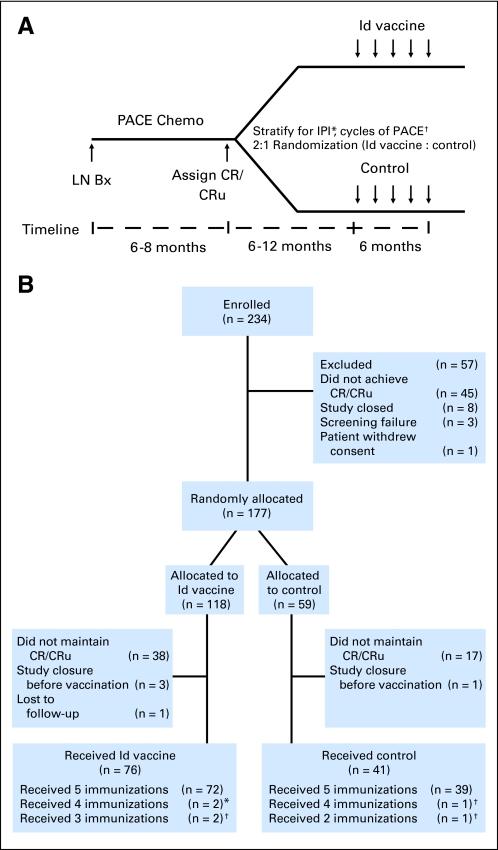
Fig 1.
(A) Clinical trial schema. Previously untreated patients with advanced stage follicular lymphoma underwent lymph node biopsy (LN Bx) after enrollment and were treated with prednisone (60 mg/m2 orally daily on days 1 to 14), doxorubicin (25 mg/m2 intravenously [IV] on days 1 and 8), cyclophosphamide (650 mg/m2 IV on days 1 and 8), and etoposide (120 mg/m2 IV on days 1 and 8; PACE) chemotherapy (chemo) every 28 days. Patients achieving complete response (CR)/CR unconfirmed (CRu) were stratified according to International Prognostic Index (IPI) and number of chemotherapy cycles and randomly assigned two to one to receive five injections of hybridoma-derived autologous tumor immunoglobulin idiotype (Id) conjugated to keyhole limpet hemocyanin (KLH) and administered with granulocyte-monocyte colony-stimulating factor (GM-CSF; Id-KLH + GM-CSF) or control vaccine (KLH + GM-CSF), respectively. (*) Low, low-intermediate or high-intermediate, high groups. (†) < eight or ≥ eight cycles. (B) CONSORT diagram of enrollment, randomization, and treatment. Two hundred thirty-four patients were enrolled and 117 patients were randomly assigned to receive at least one dose of the blinded vaccine; 76 received Id vaccine and 41 received control vaccine. Patients receiving fewer than five immunizations either (*) withdrew from the study or (†) relapsed before completion.