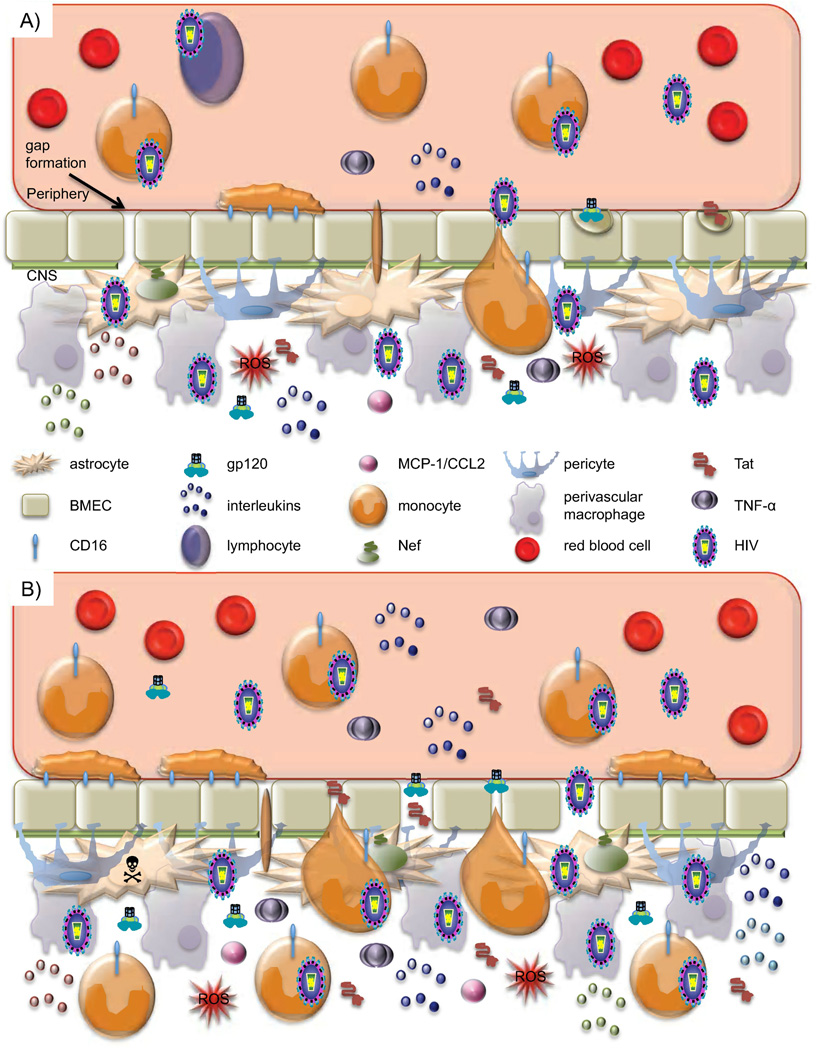
Fig. 3. Tipping point: A model of BBB dysregulation and loss of homeostasis.
A) During acute infection, an initial viral entry event into the CNS occurs. This is believed to be through entry of an infected cell of the monocyte-macrophage lineage. Throughout chronic infection, increased generation of or differentiation to a specific monocyte subset, CD14low/CD16+ that traffics preferentially to the BBB and constitutively secretes IL-1 and TNF-alpha is observed in patients with HIV. Once at the BBB, infected cells secrete IL-1, TNF-alpha, Tat, gp120 and other viral proteins causing altered TJ protein expression and localization and BMEC activation leading to increased passage across the BBB. Crosstalk between monocytes and BMECs in this inflammatory state leads to reciprocal activation of the two cell types, allowing for diapedesis of, likely infected, monocytes. Tat and gp120 are able to enter BMECs through adsorptive endocytosis, altering TJ regulation and delaying TJ closure, creating an opportunity for free virus and viral proteins to enter the CNS through non-specific passage mechanisms. In addition, gap formation has been observed under these conditions, however, the endothelium is able to repair. In the CNS, other cells including astrocytes, pericytes, and perivascular macrophages can be infected and will further secrete Tat, gp120, and proinflammatory cytokines leading to further BMEC activation. Additionally, astrocytes will express high levels of Nef, and secrete MCP-1/CCL2, IL-2, 6, and 8. This process greatly facilitates viral entry into the CNS. Additionally, Tat has been shown to be chemotactic for monocytes, bringing more monocytes to the site of infection. While homeostasis is strained, as long as the barrier is able to recover and repair balance can be restored. This state of enhanced passage may correlate with early HAND development. B) After prolonged exposure to viral proteins, proinflammatory cytokines, and infectious virus, BBB dysregulation exceeds the threshold of what can be restored. Constant BMEC activation leads to greatly increased monocyte firm adhesion and diapedesis, further spreading infection throughout the CNS. High levels of Tat and gp120 entering BMECs altering TJ protein expression and stability cause further delays in TJ closing, and there is more leakiness in passage. Gap formation is also enhanced, pushing repair mechanisms to a limit. Astrocyte death is also observed, often in cells neighboring those that are infected. Disturbance of the homeostasis between the periphery and the CNS, along with peaks in inflammatory cytokines, neurotoxic viral proteins, and infectious virions suggest a correlation with the onset of more severe neurocognitive symptoms.