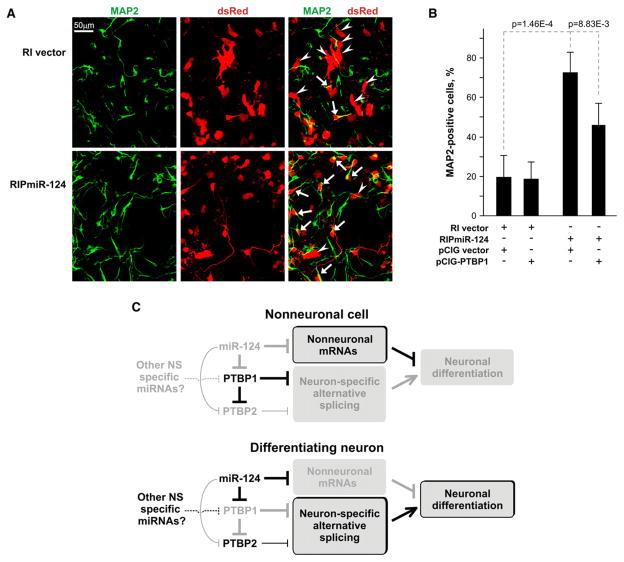
Figure 7. miR-124 Stimulates Neuronal Differentiation at Least in Part by Reducing PTBP1 Levels.
(A) miR-124 stimulates neuronal differentiation of P19 cells. Cells were transfected with RIPmiR-124 plasmid or RI vector, induced with RA for 4 days, and plated on polylysine/laminin-coated coverslips. Two days after plating, the cells were fixed and stained with antibodies against MAP2. Arrows, dsRed-positive cells, which are also MAP2 positive (neurons); arrowheads, dsRed-positive cells, which do not express MAP2 (immature neurons and glia).
(B) PTBP1 downregulation is required for an optimal stimulatory effect of miR-124. The experiment was carried out as in (A), but cells were cotransfected with RIPmiR-124 plasmid or RI vector and pCIG vector or pCIG-PTBP1 expression plasmid prior to RA treatment. Both pCIG and pCIG-PTBP1 plasmids also encode EGFP protein that localizes to the nucleus. The fraction of MAP2-positive cells among cells expressing both dsRed and EGFP was averaged over seven randomly selected fields ± SD.
(C) Regulatory networks controlled by miR-124. In nonneuronal cells, high levels of PTBP1 repress NS-specific alternative splicing, which helps maintain a nonneuronal state. During neuronal differentiation, high levels of miR-124 downregulate PTBP1, leading to a switch to NS-specific alternative splicing and neuronal differentiation. A decrease in the PTBP1 levels also promotes exon inclusion in PTBP2 mRNA and accumulation of PTBP2 protein. PTBP2 fine-tunes the splicing pattern by weakly repressing the inclusion of a subset of NS-specific exons. In addition to PTBP1, miR-124 also downregulates several other nonneuronal mRNAs, including those of SCP1, laminin γ1, and integrin β1 (Cao et al., 2007; Visvanathan et al., 2007), as well as proteins involved in cell proliferation (Figure S5; Wang, 2006).