Fig. 5.
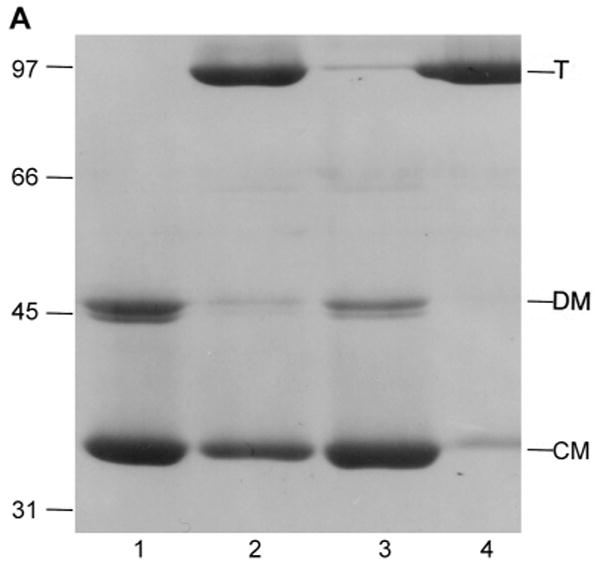
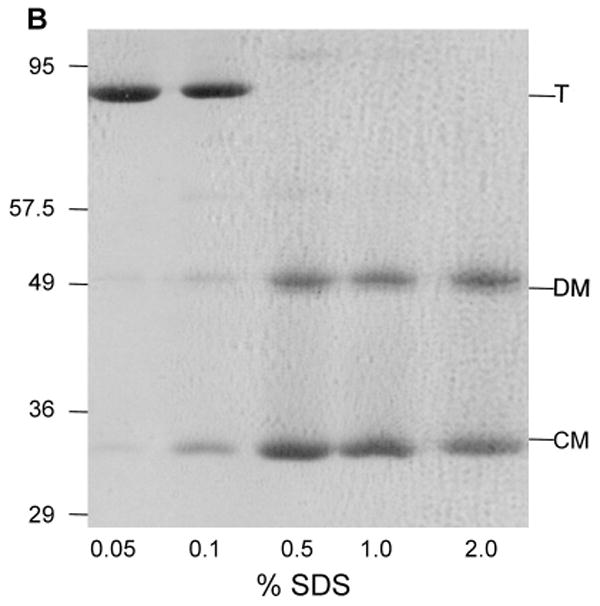
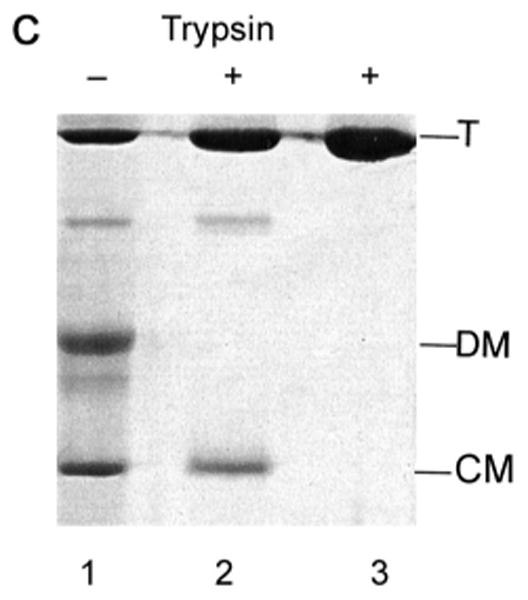
Characterization of low pH LamB monomer by SDS PAGE.
A. Dissociation of LamB protein trimer by dialysis at low pH is reversed by neutralization. Purified LamB protein (0.5 mg) was dialyzed overnight against 100 ml of 20 mM phosphate - 20 mM glycine, pH 2.5, 1% octylPOE or against 100 ml of 20 mM phosphate - 20 mM citrate, pH 3.5, 1% octylPOE. Aliquots were withdrawn and neutralized by addition of NaOH where indicated. All samples were loaded on to the gel without heat treatment. Lane 1, LamB dialyzed to pH 2.5; Lane 2, LamB neutralized after dialysis at pH 2.5; Lane 3, LamB dialyzed to pH 3.5; Lane 4, LamB neutralized after dialysis at pH 3.5. Mr of standards are indicated in kDa, and the positions of trimer (T), denatured monomer (DM) and compact monomer (CM) are marked.
B. Dissociation of LamB protein was shown to be a function of SDS concentration during SDS PAGE by varying the percent SDS in the sample buffer. The resolving gel in this case is 10% acrylamide. Samples containing 12 μg of purified LamB protein were treated with 33 mM HCl, then added to an equal volume of sample buffer containing no reducing agent and a varying amount of SDS. The concentrations of SDS in the sample are: Lane 1, 0.05%; Lane 2, 0.1%; Lane 3, 0.5%; Lane 4, 1.0%; and Lane 5, 2.0%. All samples were loaded on to the gel without heat treatment. The positions of molecular weight standards from a noncommercial mixture (see section 2.3) are shown. In addition, trimer (T), denatured monomer (DM) and compact monomer (CM) are indicated.
C. The resistance of the compact monomer to digestion by trypsin was determined with a stored sample of purified LamB protein that had been dialyzed overnight into 20 mM phosphate-20 mM glycine buffer, pH 2.6, with 0.5% oPOE. After storage at 4°C, it was again dialyzed to 10 mM phosphate-10 mM glycine, pH 7.2, 0.5% oPOE. Both samples, each containing 42 μg of LamB protein, along with untreated LamB protein (pH 7.2) were treated with 1 μg trypsin 30 min at 37°C and then 3 μg of trypsin inhibitor was added. SDS PAGE was carried out without heat treatment. Lane 1 and 2 are samples dialyzed back to pH 7.2 after storage with and without trypsin. Lane 3 is native LamB with trypsin. The positions of trimer (T), denatured monomer (DM) and compact monomer (CM) are shown.
