Figure 4.
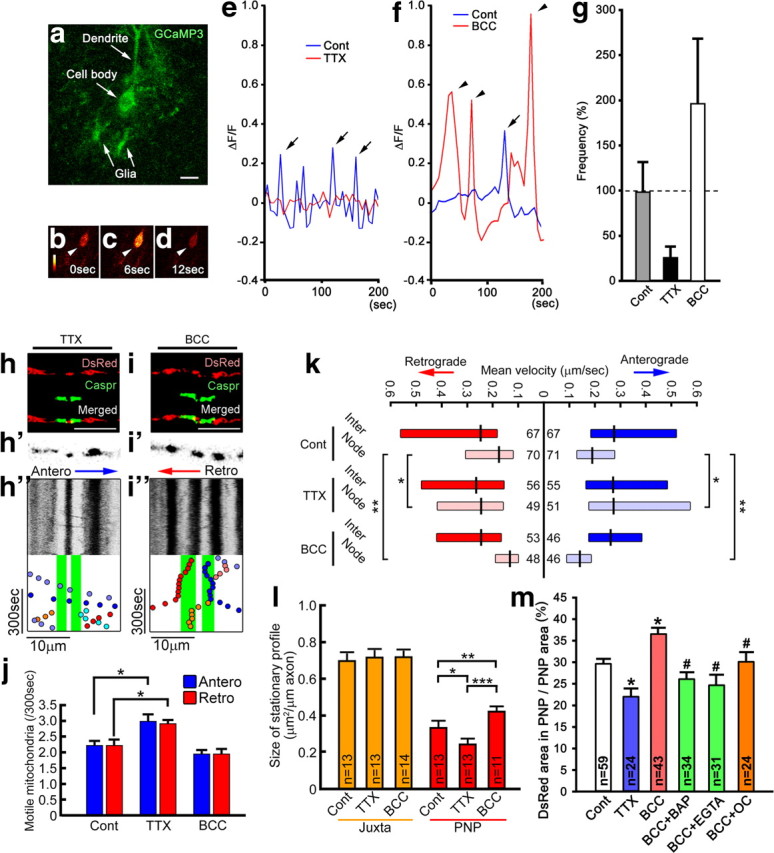
Electrical activity and Ca2+ increase the size of mitochondrial stationary sites and decrease mitochondrial motility in nodal axoplasm. Mitochondrial transport speed and distribution were investigated following TTX and BCC treatment. a–g, In a Purkinje cell body expressing GCaMP3 (a), increase (b to c, arrowheads) and return (c to d, arrowheads) of fluorescent intensity show a Ca2+ transient. The frequency (and amplitude) of Ca2+ transients are decreased threefold by TTX (e) and increased twofold (f) by BCC (g). h–j, Kymographs of nodal axoplasm after TTX (h–h″) and BCC (i–i″) show the number of motile mitochondria is increased by TTX and unchanged by BCC (j). *p < 0.01; n = 18 axons for each group. k, The speed of mitochondrial transport was significantly increased by TTX and significantly decreased by BCC in nodal axoplasm but not in internodal axoplasm. *p = 0.03, **p < 0.001; n, number of mitochondria marked on each bar. l, TTX decreased and BCC increased mitochondrial stationary site size in nodal axoplasm, but not in juxtaparanodal axoplasm. *p = 0.02, **p = 0.01, ***p < 0.001. m, TTX also decreased and BCC increased the percentage nodal axoplasm area occupied by mitochondria. Ca2+ chelaters [EGTA, BAPTA-AM (BAP)] or a Ca2+ channel blocker [ω-conotoxin MVIIC (OC)] eliminated the increase of nodal mitochondria size by BCC. #p < 0.05 compared with BCC alone, *p < 0.001; n = number of fibers (l) or nodes (m) examined. g, j, l, m, Bars, Mean + SEM. k, Boxes, Median with first and third quartiles. Cont, control; PNP, nodal–paranodal; Inter, internodal; Juxta, juxtaparanodal; Antero, anterograde; Retro, retrograde.
