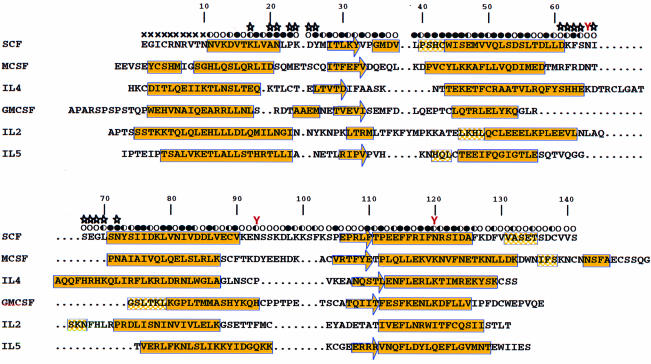
Fig. 3. Structure-based sequence alignment of SCF with other short-chain helical cytokines of human species. Dots denote gaps. The secondary structure elements were assigned according to the output of the PROCHECK program (Laskowski et al., 1993) except the helix assignment for residues 35–38, which was identified by inspection of the hydrogen bond pattern. Secondary structures are shown in yellow with filled boxes referring to α-helices, half-filled boxes to 310-helices and arrows to β-strands. The solvent accessibility of the SCF dimer is indicated for each residue by an open circle if the fractional solvent accessibility is >0.4, a half-filled circle if it is 0.1–0.4, and a filled circle if it is <0.1. Residues at the SCF dimer interface are identified by stars, and the N-linked glycosylation sites by red Ys above the Asn residues.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.