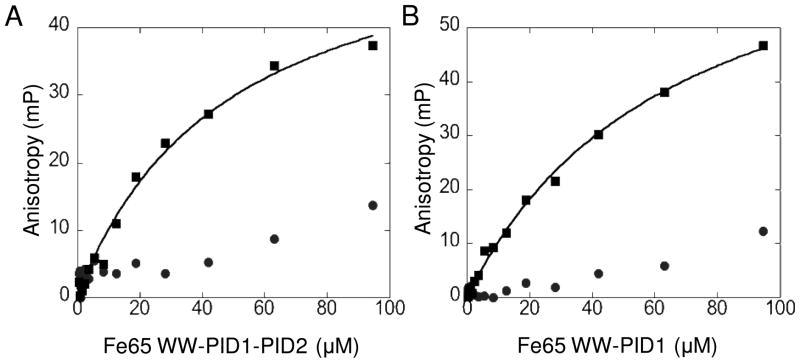
Figure 6.
Fluorescence anisotropy of LRP4488 or pYLRP4488 binding to Fe65. LRP4488 (●) or pYLRP4488 (■) conjugated to Oregon Green 488 maleimide (Invitrogen) was incubated with 0.1–100 μM Fe65 WW-PID1-PID2 236-662 (A) or 0.1–100 μM Fe65 WW-PID1 236-512 (B). Anisotropy values at each Fe65 concentration were determined using a DTX 880 Multimode Dectector Beckman Coulter plate reader with excitation filter at 485 nm and two emission filters at 535 nm equipped with polarizers. Data were fit with KaleidaGraph 4.0 software using a Michaelis-Menten curve fit. pYLRP4488 bound Fe65 WW-PID1-PID2 with KD = 48.3+/−7.3 μM and pYLRP4488 bound Fe65 WW-PID1 with KD = 66.7 +/− 6.2 μM.