Summary
Schizophrenia was associated with a distinct autosomal abnormality in two related mildly dysmorphic individuals. The finding of cosegregation of schizophrenia and a partial trisomy of chromosome 5 in the family suggests a potential location of a gene or genes linked to schizophrenia.
INTRODUCTION
Schizophrenia affects 0·5–1% of the population11,2 Although the syndrome clusters in families the inheritance pattern is complex and does not follow Mendelian models.3 Specific gene involvement has not yet been proven. We report here some findings that may point to the location of such a gene.
SUBJECTS AND INVESTIGATIONS
The family studied is of Asian descent, living in Vancouver, Canada. Two members of the family have schizophrenia—a 20-year-old male college student and his 52-year-old maternal uncle. The 20-year-old proband was admitted to inpatient psychiatric care with typical symptoms of acute schizophrenia (prominent auditory hallucinations and paranoid and bizarre delusions of four weeks’ duration). He had had no change in mood and there was no alcohol or drug history. He had deteriorated in social functioning and school performance and had had prodromal symptoms for several years. He met both International Classification of Disease (ICD-94) and DSM.-III-R5 criteria for schizophrenia. His psychotic symptoms resolved with low-dose neuroleptic treatment (2 mg haloperidol daily). He subsequently had residual symptoms of low motivation, blunted emotions, and poverty of speech.
The proband was noted on admission to have slightly different facial features from his parents. The mother remarked he looked similar to her brother who also had schizophrenia. This maternal uncle had had first onset of social withdrawal, paranoid delusions, and auditory hallucinations without prominent mood change at age 20. The psychosis had relapsed on several occasions, responding each time to low-dose neuroleptic treatment which he continues to take (100 mg chlorpromazine daily). He still has blunted emotions and low motivation, and meets ICD-9 and DSM-III-R criteria for chronic schizophrenia.
Because of the conjunction of schizophrenia and subtle dysmorphic facial features the two men were examined thoroughly for other physical abnormalities and they proved to have the following constellation of structural abnormalities and features of schizophrenia: frontal bossing; flat occiput; hypertelorism (widely spaced eyes); overfolded protuberant ears; short stature (<3rd percentile); mild obesity; short 4th proximal phalanx (toe); partial syndactyly fingers and toes (soft tissue fusion); left kidney abnormal on ultrasound examination, possibly duplex (left kidney absent in proband’s maternal uncle); small phallus (< 10th percentile); full syndrome of schizophrenia meeting narrowly defined classification, with onset of acute psychosis at age 20 and responsiveness to low-dose neuroleptics. Neither individual had evidence of neurological impairment or mental retardation. No other family members have similar physical features or any history of mental illness.
Cytogenetic studies revealed that the two men had an identical unbalanced male karyotype with extra chromosomal material in the long arm of chromosome 1 (fig 1). Chromosome analysis of the phenotypically normal mother of the proband showed the same abnormal chromosome 1 and one chromosome 5 with a deletion in the proximal long arm. Since the size and banding pattern of the additional material inserted into chromosome 1 correspond with those of the material missing from chromosome 5, the mother seems to carry a balanced insertion (fig 2). Both the proband and his uncle have inherited the same derivative chromosome 1 from a balanced 1;5 insertion carrier parent (the parents of the uncle were not available for karyotyping) along with a normal pair of chromosomes 5. Consequently both are trisomic for the 5q11.2 to 5q13.3 segment. There was no evidence for any other gain or loss of chromosomal material in their karyotypes. The karyotypes of all other living members of the pedigree were normal (fig 3).
Fig 1. Partial G-banded karyotype and ISCN20 idiogram of chromosomes 1 and 5 of the proband (identical to that of the affected maternal uncle).
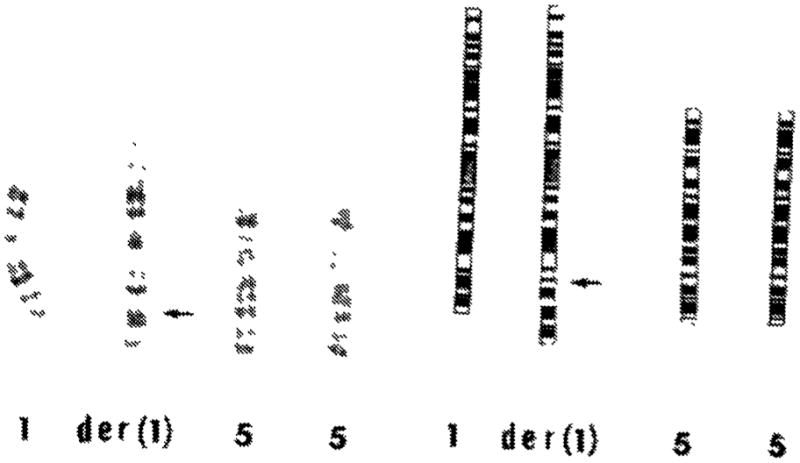
Both chromosomes 5 are intact. One of the chromosomes 1 [der(1)] shows insertion of the extra 5q11.2 to 5q13.3 segment into its long arm at the band lq32.3, indicated by the arrow. The karyotype is 46,XY, der(1) invins (1;5)(q32.3;q13.3q11.2). It contains three copies of the proximal 5q segment 5q11.2 to 5q13.3.
Fig 2. Partial G-banded karyotype and ISCN idiogram (chromosomes 1 and 5) of the proband’s mother.
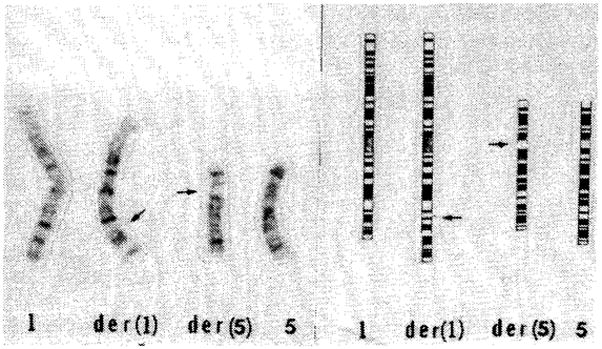
There is a balanced insertion of a proximal 5q segment into the long arm of chromosome 1 [der(1)]. High resolution studies suggest that the inserted segment had also inverted. The 5q arm of the involved chromosome [der (5)] has a deletion of the 5q11.2 to 5q13.3 segment indicated by the arrow. Detailed analysis of all other chromosomes revealed no structural rearrangements at the 400–500 band level. The maternal karyotype is 46,XX, invins (1;5) (q32.3;q13.3q11.2)?mat?pat.
Fig 3. Family pedigree showing cosegregation of schizophrenia, physical abnormalities, and a partial trisomy of chromosome 5.
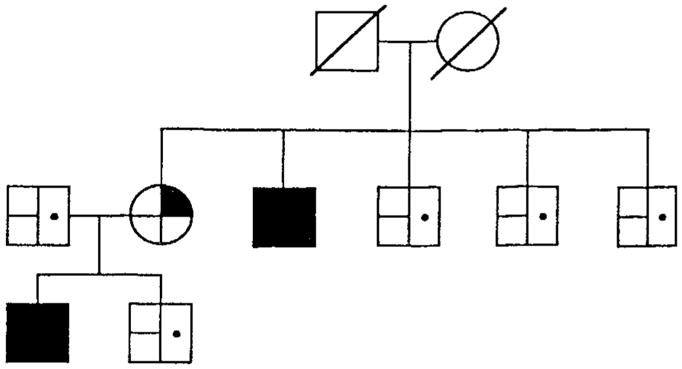
 partial trisomy chromosome 5;
partial trisomy chromosome 5;
 balanced translocation carrier;
balanced translocation carrier;
 normal chromosomes;
normal chromosomes;
 schizophrenia;
schizophrenia;
 no mental illness;
no mental illness;
 physical abnormalities;
physical abnormalities;
 no physical abnormalities.
no physical abnormalities.
DISCUSSION
Although some genetic conditions have been associated with “schizophrenia-like psychoses”,6 this is the first report of a distinct autosomal abnormality in individuals with a narrowly defined schizophrenic syndrome. The schizophrenic disorder of these patients is typical in acute psychotic symptoms, onset, course, and neuroleptic response, and is not complicated by mental retardation. The remarkable feature in these two men is that they have a strikingly similar schizophrenic phenotype and structural anomalies. The most parsimonious explanation for these findings is that the abnormalities, including the schizophrenic illness, arise from their shared chromosomal abnormality. The proband’s mother is a carrier of the balanced inverted insertion but she has no evidence of the mental disorder nor die physical characteristics of her son or brother. This suggests that it is not the disruption of chromosome 1 and rearrangement of chromosome 5 but rather the partial trisomy—that is, the presence of three copies of the chromosome 5 segment (5q11.2 to 5q13.3)—that is causing the physical abnormalities and schizophrenia. This could also be a chance association, although no other family members have the partial trisomy, mental illness, or the distinctive physical features.
Genetic factors are important in the aetiology of schizophrenia. However, the precise pattern and mode of inheritance is unknown.3 Recessive, dominant with incomplete penetrance, and multigenic patterns have each been postulated from pedigree and statistical analyses.7–9 There seems to be no linkage with common markers (HLA, Gc antigens).10,11 Genetic linkage studies with molecular DNA markers have been used to localise a major gene for manic depressive illness to chromosome 1112 and another to the X chromosome.13 Similar studies of familial Alzheimer’s disease have revealed genetic linkage to chromosome 21.14 The observed association between Down syndrome (trisomy 21) and Alzheimer’s disease (AD) provided a clue to the location of an AD gene.15 While it is now possible to test almost the entire genome for linkage by means of molecular DNA markers,16 such a study would require much greater resources than a test of one particular candidate region. The subject family provides the first clue to link schizophrenia to a specific region of the human genome. While this finding is consistent with direct involvement of chromosome 5 in the aetiology of schizophrenia, genes in the trisomic area could cause the illness indirectly by affecting loci on other chromosomes. Also, schizophrenia is likely to be a heterogeneous disorder. Nevertheless, it will be possible to use DNA probes located within the candidate trisomic region of chromosome 5 to detect restriction fragment length polymorphisms which coinherit with schizophrenia in large-scale pedigree studies.17–19 If such studies find linkage it may become possible to isolate a major gene that predisposes to schizophrenia.
Acknowledgments
This work was supported by the National Institute of Mental Health, grant 1R03 MH 43730. We thank Dr Charles A. Kaufmann for comments on the paper.
References
- 1.Robins LN, Helzer JE, Weissman MM, Orvaschel H, Gruenberg E, Burke JD, Jr, Regier DA. Lifetime prevalence of specific psychiatric disorders in three sites. Arch Gen Psychiatry. 1984;41:949–58. doi: 10.1001/archpsyc.1984.01790210031005. [DOI] [PubMed] [Google Scholar]
- 2.Bleuler M. The schizophrenic disorders: long-term patient and family studies. New Haven: Yale University Press; 1978. [Google Scholar]
- 3.Gottesman II, Shields J. Schizophrenia: the epigenetic puzzle. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- 4.World Health Organization. ICD 9th revision. Vol. 1. Geneva: WHO; 1978. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- 6.Propping P. Genetic disorders presenting as “schizophrenia”. Karl Bonhoeffer’s early view of the psychoses in the light of medical genetics. Hum Genet. 1983;65:1–10. doi: 10.1007/BF00285021. [DOI] [PubMed] [Google Scholar]
- 7.Risch N, Baron M. Segregation analysis of schizophrenia and related disorders. Am J Hum Genet. 1984;36:1039–59. [PMC free article] [PubMed] [Google Scholar]
- 8.McGue M, Gottesman, Rao DC. The transmission of schizophrenia under a multifactorial threshold model. Am J Hum Genet. 1983;35:1161–78. [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci USA. 1967;58:199–205. doi: 10.1073/pnas.58.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGue M, Gottesman II, Rao DC. The analysis of schizophrenia family data. Behav Genet. 1986;16:75–87. doi: 10.1007/BF01065480. [DOI] [PubMed] [Google Scholar]
- 11.McGuffin P, Sturt E. Genetic markers in schizophrenia. Hum Hered. 1986;36:65–88. doi: 10.1159/000153604. [DOI] [PubMed] [Google Scholar]
- 12.Egeland JA, Gerhard DS, Pauls DL, et al. Bipolar affective disorders linked to DNA markers on chromosome 11. Nature. 1987;325:783–87. doi: 10.1038/325783a0. [DOI] [PubMed] [Google Scholar]
- 13.Baron M, Risch N, Hamburger R, et al. Genetic linkage between X-chromosome markers and bipolar affective illness. Nature. 1987;326:289–92. doi: 10.1038/326289a0. [DOI] [PubMed] [Google Scholar]
- 14.St George-Hyslop PH, Tanzi RE, Polinsky RJ, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235:885–90. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- 15.Schweber M. A possible unitary genetic hypothesis for Alzheimer’s disease and Down syndome. Ann NY Acad Set. 1985;450:223–38. doi: 10.1111/j.1749-6632.1985.tb21495.x. [DOI] [PubMed] [Google Scholar]
- 16.Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980;32:314–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Weissman MM, Merikangas KR, John K, Wickramaratne P, Prusoff BA, Kidd KK. Family-genetic studies of psychiatric disorders. Developing technologies. Arch Gen Psychiatry. 1986;43:1104–16. doi: 10.1001/archpsyc.1986.01800110090012. [DOI] [PubMed] [Google Scholar]
- 18.Gurling H. Application of molecular biology to mental illness Analysis of genomic DNA and brain mRNA. Psychiatr Dev. 1985;3:257–73. [PubMed] [Google Scholar]
- 19.Baron M. Genetics of schizophrenia. II Vulnerability traits and gene markers. Biol Psychiatry. 1986;21:1189–211. doi: 10.1016/0006-3223(86)90225-8. [DOI] [PubMed] [Google Scholar]
- 20.Standing Committee on Human Cytogenic Nomenclature. An international system for human cytogenetic nomenclature. Basel: Karger; 1985. [Google Scholar]


