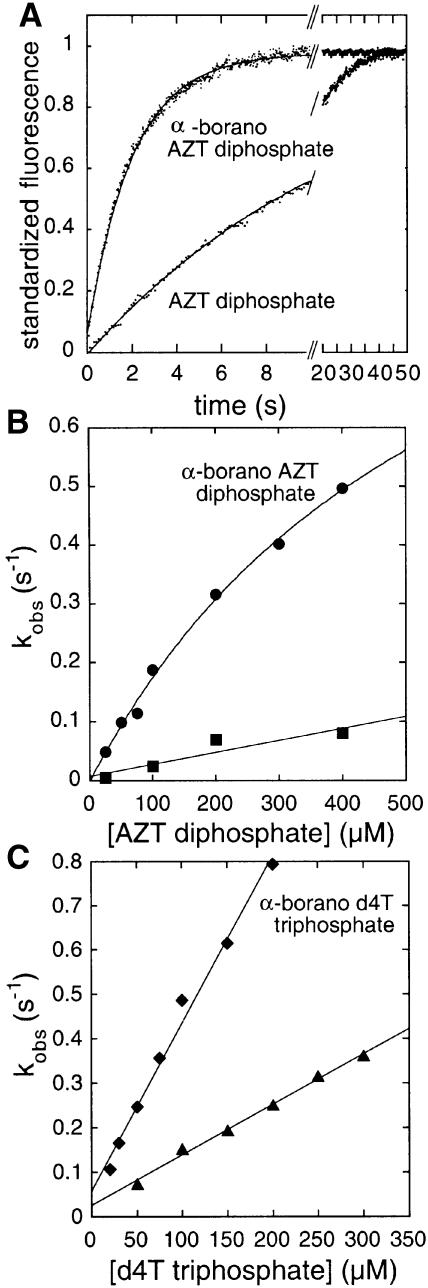
Fig. 3. Enhancement of AZT and d4T activation by NDPK in the presence of the Rp α-borano group. (A) Kinetics of phosphorylation of AZT diphosphate and α-borano-AZT diphosphate by phosphorylated human NDPK A. The phosphorylated enzyme (1 µM), prepared as described (Deville-Bonne et al., 1996), is mixed with 400 µM AZT diphosphate or α-borano-AZT diphosphate in 50 mM Tris–HCl pH 7.5, 5 mM MgCl2, 75 mM KCl, 1 mM DTT and 5% glycerol (final concentrations) at 20°C. The decrease in fluorescence is monitored with a Hi-Tech SF-61DX2 stopped flow device (λexc = 304 nm, excitation slit = 2 nm, emission filter <320 nm). The solid lines represent the best fit of each curve to a monoexponential. (B) Concentration dependence of the pseudo-first order phosphorylation rate constant kobs: α-borano-AZT diphosphate (circles) and AZT diphosphate (squares). Data were analysed according to the classical model of a fast binding reaction followed by a slow, rate-limiting phosphotransfer (Schneider et al., 1998). The catalytic efficiency is kmax/KD = 2000 M–1s–1 for α-borano-AZT diphosphate and 200 M–1s–1 for AZT diphosphate. As no saturation is reached, the maximum rate of phosphotransfer, kmax, is not measurable in this experiment. (C) Concentration dependence of the pseudo-first order rate constant kobs for NDPK A phosphorylation by α-borano-d4T triphosphate (diamonds) and d4T triphosphate (triangles). The catalytic efficiency is kmax/KD = 6000 M–1s–1 for α-borano-d4T triphosphate and 800 M–1s–1 for d4T triphosphate.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.