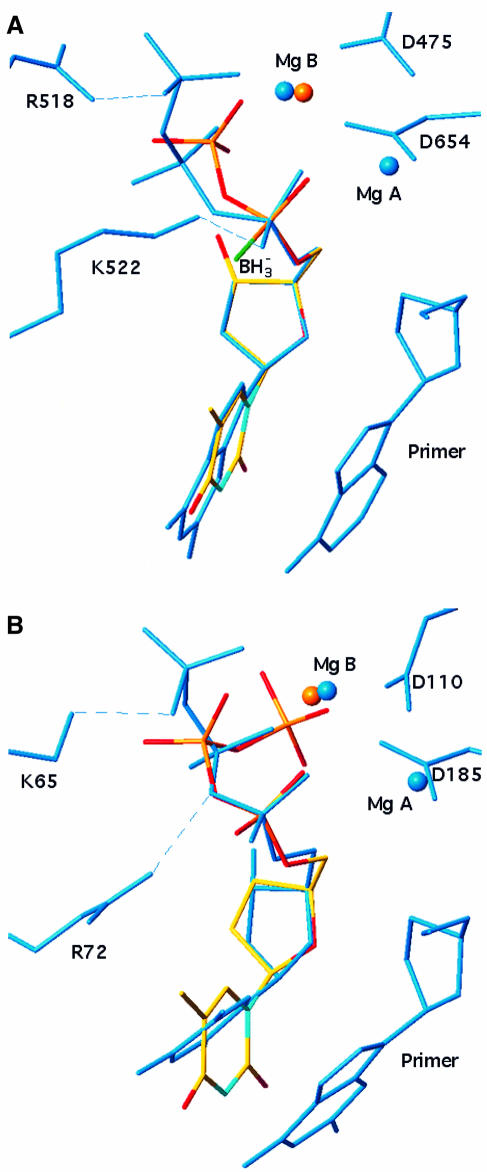
Fig. 4. Conformation of the nucleotide substrate in NDPK, T7 DNA polymerase and HIV reverse transcriptase. d4T triphosphate from the NDPK complex in Figure 2B is shown in atom-type coloured bonds superimposed onto (A) dideoxyGTP in the ternary complex with bacteriophage T7 DNA polymerase–DNA (PDB file 1T7P) and (B) deoxyTTP in the ternary complex with HIV reverse transcriptase–DNA (PDB file 1RTD). Least-square fitting was performed on atom N1 of the base and common atoms in the sugar and the α-phosphate. In T7 polymerase and reverse transcriptase, relevant active site residues, DNA and the ligand are in blue bonds, and blue spheres represent two Mg2+ ions bound. In NDPK, the red sphere is the single Mg2+ ion bound to d4T triphosphate. It is located <1 Å away from one of the two Mg2+ of the polymerases.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.