Abstract
Myasthenia gravis (MG) is caused by antibodies that react mainly with the acetylcholine receptor on the postsynaptic site of the neuromuscular junction. A wide range of clinical presentations and associated features allow MG to be classified into subtypes based on autoantibody status. Striational antibodies, which react with epitopes on the muscle proteins titin, ryanodine receptor (RyR), and Kv1.4, are frequently found in MG patients with late-onset and thymoma. Antititin and anti-RyR antibodies are determined by enzyme-linked immunosorbent assay or immunoblot. More recently, a method for the detection of anti-Kv1.4 autoantibodies has become available, involving 12–15% of all MG patients. The presence of striational antibodies is associated with more severe disease in all MG subgroups. Anti-Kv1.4 antibody is a useful marker for the potential development of lethal autoimmune myocarditis and response to calcineurin inhibitors. Detection of striational antibodies provides more specific and useful clinical information in MG patients.
1. Introduction
Acquired myasthenia gravis (MG) in an organ-specific autoimmune disorder generally mediated by antiacetylcholine receptor (AChR) or less frequently by antimuscle-specific tyrosine antibodies at the neuromuscular junction [1]. Some MG patients have antibodies that bind in a cross-striational pattern to skeletal and heart muscle tissue sections. They were known as “striational antibodies.” These autoantibodies recognize epitopes on skeletal muscle proteins including myosin, actin, actinin, and filamin [2–5]. Particularly, three types of striational antibodies including those to titin, ryanodine receptor (RyR), and Kv1.4 have been investigated by many researchers. The detection of these three striational antibodies can provide more specific clinical information and are associated with the subtypes of MG patients. In this article, we describe the characteristics of these three types of striational antibodies.
2. Molecular Structure
Titin is a giant protein (3000 kD) abundantly in the skeletal and cardiac sarcomere. Ninety percent of the titin mass is contained in a repetitive structure of 2 different 100-residue repeats [6]. Anti-titin antibody was first discovered in the serum of MG patients by Aarli et al. in 1990 [7]. Autoantibodies to titin are now determined by a commercially available enzyme-linked immunosorbent assay (ELISA). The main immunogenic region of titin is called myasthenia gravis titin-30 (MGT-30) and is situated near the A/I-band junction [8–10].
RyR is a calcium release channel located in the sarcoplasmic reticulum. There are two forms of RyR, skeletal (RyR1) and cardiac (RyR2). The RyR is a protein containing 5035 amino acids with a molecular weight of 565 kD. It is composed of 4 homologous subunits that can build a tetramer with a central channel [8]. Anti-RyR antibody was first identified by Mygland et al. in 1992 using western blot for the presence of antibodies to the protein of the sarcoplasmic reticulum from rabbit skeletal muscle [11]. Although cardiac and skeletal muscle RyRs are antigenically different, anti-RyR antibodies in MG patients cross-react with both subtypes of the receptor [12]. Several epitopes in both the N- and C-terminus of RyR1 sequence are identified and used as antigenic peptide in ELISA.
Voltage-gated K channel (VGKC) consists of four transmembrane α-subunits that combine as homo- or heterotetramers. Kv1.4 is an α-subunit with a molecular weight of 73 kD located mainly in the brain, peripheral nerves, and skeletal and heart muscles. Anti-Kv1.4 antibody was first discovered by our group in 2005 using a protein immunoprecipitation assay using 35S-labeled rhabdomyosarcoma (RD) cellular extracts [13]. We cannot detect anti-Kv1.4 antibody by immunoblot or ELISA using Kv1.4 recombinant protein. This finding suggests that conformational epitopes may be necessary for the detection of anti-Kv1.4 antibody.
3. Antibodies Detection
MG can be classified into several subtypes based on the autoantibodies profile [1, 8]. Striational antibodies are principally detected only in the sera of MG patients, but not in healthy or diseased controls. Striational antibodies are rarely found in AChR antibody-negative MG. The seropositivity of striational antibodies was different in the examined populations. Generally, anti-titin antibody is detected in 20–40% of all MG patients, anti-RyR in 13–38%, and anti-Kv1.4 in 12–15% [8, 14–19]. It is well known that striational antibodies are associated with the late-onset MG subgroup. The disease onset age is eldest in MG patients with anti-titin antibodies and youngest in those with anti-Kv1.4 antibodies [8, 14–19]. It is likely that the gender ratio is almost equal in striational antibodies.
Anti-titin antibodies are closely associated with older-onset MG, and 60–80% of MG patients at disease onset older than 60 years have anti-titin antibodies [8, 14–17, 19]. Our recent study showed that 32% of late-onset MG cases without thymoma were positive for anti-titin antibodies when the cutoff age between early- and late-onset MG was defined as 50 years [20, 21]. In addition, there can be two or three of striational antibodies in a single MG patient. When we measured anti-AChR, anti-titin, and anti-Kv1.4 antibodies in 209 Japanese MG patients, we found 8 MG patients who were positive for all three autoantibodies [19].
To date, anti-titin and anti-RyR antibodies have been examined in many MG patients in the US, Europe, and Asian countries. Since common characteristics of MG patients with anti-titin and anti-RyR antibodies have been described, the clinical picture may be common across different ethnic groups and immunogenetic backgrounds. On the other hand, since anti-Kv1.4 antibodies were studied only in Japan, further examination is necessary in Caucasian MG patients.
4. Immunopathogenesis
There is no evidence that striational antibodies can really induce structural changes in skeletal muscle [8]. However, striational antibodies potentially indicate the presence of a pathological process that, in addition to the AChR antibody-mediated NMJ transmission defect, influences the muscle cell function of the patient.
Some immunological evidence including complement activation by striational antibodies and T cell proliferative response to MGT-30 have been reported [22–24]; however, these results do not prove the existence of any pathogenic role for striational antibodies in MG. The presence of titin antibodies in patients with MG correlates with the electromyographic evidence of myopathy [25]. Anti-titin antibody is associated with HLA DR7 in Caucasian MG patients [8, 15, 21]. DR3 and DR7 have opposing effects on MG phenotypes in Caucasians. DR3 has a positive association with early-onset MG and a negative association with late-onset MG, and DR7 has the opposite association [1]. DR3 and DR7 are very rare in Japanese populations. In contrast, DR9 and DR2 in Japanese MG patients have similar associations to those of DR3 and DR7, respectively, in Caucasian patients [20].
Anti-RyR antibodies cause allosteric inhibition of RyR function in vitro, inhibiting Ca2+ release from the sarcoplasmic reticulum [26]. Some MG patients have been shown to have impaired excitation-contraction (E-C) coupling in addition to neuromuscular transmission failure [27, 28]. The mechanisms of E-C coupling are closely related to RyR function, and anti-RyR antibody may influence muscle contraction. In this regard, a 27-year-old MG patient, who was positive for anti-RyR antibody, but not anti-AChR, was proven to be impaired E-C coupling [29]. In addition to anti-RyR antibody, autoantibodies against dihydropyridine receptor or transient receptor potential canonical type 3, which have functional interactions with RyR1 in Ca2+ release, were also detected in MG patients [30, 31].
On the other hand, antineuronal VGKC antibody is principally different from anti-Kv1.4 antibody (muscular VGKC). Autoantibodies to neuronal VGKC are known to be associated with acquired neuromyotonia, Morvan's syndrome, and autoimmune nonparaneoplastic limbic encephalitis. The sera of patients with these diseases mainly target the Kv1 α-subunits: Kv1.1, Kv1.2, or Kv1.6. The expression of these subunits that form Kv channels differs between the brain and muscle. Recently, the clinical spectrum of neurologic manifestations associated with neuronal VGKC autoimmunity has been expanding [32]. However, leucine-rich, glioma-inactive protein 1 was identified as a novel autoantigen in limbic encephalitis previously attributed to neuronal VGKC [33].
5. Thymoma-Associated MG
Most patients with thymoma-associated MG (T-MG) demonstrate an antibody profile with a broad striational antibody response [1, 8]. The presence of thymoma is thought to worsen the prognosis of MG, as symptoms are usually severe in these patients and are not significantly improved by thymectomy [34]. Of the 260 MG patients in our institutions, 62 (24%) had thymoma. The onset age of the thymomatous MG patient was 47 ± 12 years [19]. Bulbar involvement and myasthenic crisis were more common in patients with T-MG than in those without thymoma.
The frequencies of striational antibodies in T-MG patients are generally high. Many reports have shown that anti-titin antibodies are detected in 49–95% of T-MG, with anti-RyR found in 70–80% of cases and anti-Kv1.4 in 40–70% of cases [7, 8, 11–20]. A high frequency of striational antibodies in T-MG contributes to severe MG symptoms. Titin and RyR epitopes have been identified in thymoma tissue [35–38]. In addition, we also confirmed that Kv1.4 mRNA was detected in thymoma tissue [13]. It is generally believed that the aberrant immunization of T-cell against autoantigens is promoted by the pathogenic microenvironment inside the thymoma [1, 8]. IgG striational autoantibodies to titin are also produced by clonal thymic B cells established from patients with T-MG [39]. In addition, the autoantibodies for C-terminal regions in RyR1 are frequently detected in T-MG and may contribute to muscle dysfunction via the impairment of Ca2+ release [40]. Anti-RyR antibodies are found in spontaneous thymoma model rats [41]. Clinically, the presence of striational antibodies and computed tomographic scans of the anterior mediastinum show a similar sensitivity for thymoma in MG patients. The presence of titin and RyR antibodies in a young patient with MG strongly suggested the presence of a thymoma [8].
6. Clinical Presentation
The presence of striational antibodies is associated with more severe disease in all MG subgroups. Many studies clearly demonstrated that disease tends to be more severe in MG patients found to be positive for each striational antibody than in those found to be negative [8, 13, 17, 18, 42–44]. When the disease severity is compared between different striational antibodies, MG patients with anti-Kv1.4 antibodies show more severe symptoms than those with anti-titin antibodies [19]. Similarly, it is also reported that MG patients with anti-RyR antibodies have more severe manifestations than those with anti-titin antibodies [18]. In anti-Kv1.4-positive patients, the frequencies of bulbar involvement and myasthenic crisis were 73% and 31%, respectively [19]. In addition, patients with anti-RyR antibodies have high rates of bulbar, respiratory, and neck involvement at MG onset [18].
The other remarkable finding is the association between striational antibodies and myositis and/or cardiomyositis concomitant with MG [45, 46]. Autoimmune-mediated myocarditis and/or myositis developed in a few patients with MG, especially thymoma-associated MG. Inflammatory myopathies did not only lead to the deterioration of muscular weakness, but were also the most serious complications in the courses of MG patients. Since the mortality of MG itself has dramatically decreased recently, cardiac involvement in MG patients may be lethal. It is well known that myocarditis is accompanied by thymoma-associated MG, known as “Herzmyathenie” [46, 47]. In this regard, Evoli et al. reported that 7 of 50 T-MG cases suffered sudden deaths [34]. They speculated that some of these cases were affected by myocarditis, although autopsy studies were not performed.
Our survey in 5 Japanese institutions showed that of 924 MG patients, 8 (0.9%) had inflammatory myopathies [46]. The onset age of MG was 55.3 ± 10.3 years. All patients showed severe symptoms with bulbar involvement accompanied by myasthenic crisis in 5 and invasive thymoma in 4. Myocarditis was found in 3 patients and myositis in 6. Myocarditis, developing 13–211 months after MG onset, was characterized by heart failure and arrhythmias. Histological findings of skeletal muscles showed CD8+ lymphocyte infiltration. Seven patients had at least one of three striational autoantibodies. Immunomodulatory therapy was required in all patients and was effective for both MG and inflammatory myopathies, except in one autopsy case.
We also confirmed that some MG patients with anti-Kv1.4 antibodies had a risk for lethal arrhythmias including ventricular tachycardia, sick sinus syndrome, and complete atrial ventricular block (Figure 1) [48, 49]. We emphasize that special attention should be paid to MG patients with anti-Kv1.4 antibodies. Although various Kv channels are expressed in cardiac muscles and implicated in electrophysiological function, the association between autoantibodies to Kv channels and arrhythmias is not fully elucidated [50]. We are now investigating the mechanisms of the cardiac involvement of Kv1.4 autoimmunity.
Figure 1.
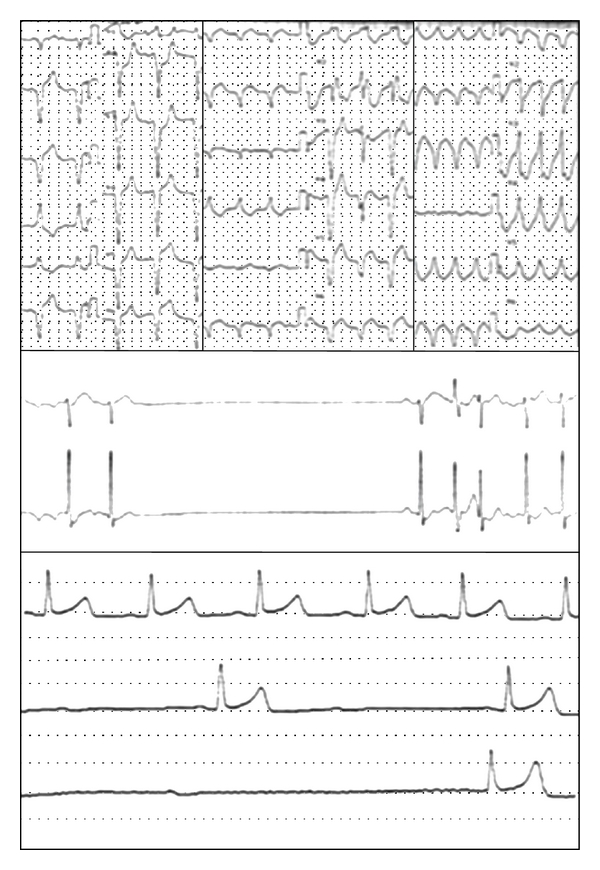
Electrocardiogram in myasthenia gravis patients with anti-Kv1.4 antibodies. (a) Ventricular tachycardia, (b) sick sinus syndrome, and (c) complete atrial ventricular block.
7. Treatment and Management
Since MG patients with striational antibodies have severe symptoms, they usually require strong immunosuppressive treatments. To avoid the many serious side effects of corticosteroids, we prefer to use a combination of low or medium doses of prednisone and other immunosuppressive agents. In Japan, two calcineurin inhibitors (CNIs), cyclosporine and tacrolimus, are widely used, since they are officially approved as medications for MG treatment under the national health insurance system in Japan. Since tacrolimus acts as an enhancer of RyR-related Ca2+ release from the sarcoplasmic reticulum, anti-RyR antibody is linked to the early pharmacological effects of tacrolimus [40]. To assess the factors associated with the response to CNIs in MG, we retrospectively analyzed the 6-month effect of CNIs in 62 MG patients [51]. Patients who achieved either a ≥3-point reduction in quantitative MG score or a ≥25% reduction in the daily dose of prednisolone were regarded as responders to CNIs. Anti-Kv1.4 antibody was proven to be associated with being a responder to CNIs.
The long-term prognosis of MG patients with striational antibodies has not been fully elucidated. Side effects of immunotherapies including infection, diabetes, stroke, ischemic heart disease, and cancers may also be more important factors in mortality associated with MG than the MG itself. The detection of striational antibodies may be potentially useful for planning therapeutic strategy. The presence of anti-RyR antibodies in T-MG and titin/RyR in nonthymoma MG indicates a less favorable prognosis [43]. However, some late-onset MG patients with anti-titin antibodies are limited to the ocular form for long periods. Further studies are necessary to prove the association between the striational antibodies and the long-term prognosis of MG.
8. Conclusion
We reviewed the characteristics of three types of striational antibodies (Table 1). Although 20 years have passed since the discovery of anti-titin antibodies in MG patient, the detection of striational antibodies is not routinely tested in the clinical management by all neurologist. Recently, several therapies for MG have emerged, including rituximab and antigen-specific apheresis whereas other treatments await clarification of efficacy and their role in MG [1]. The treatment of MG should be individualized according to clinical presentation or subtype, and requires a comprehensive assessment of the patient's functional impairment and the effect of MG on his or her daily life. The detection of striational antibodies can provide information that is useful for the classification and management of MG patients.
Table 1.
Three types of striational antibodies in myasthenia gravis (MG).
| Autoantigen | Titin | Ryanodine receptor | Voltage-gated K channel (VGKC) |
|---|---|---|---|
| (RyR) | Kv1.4 | ||
| Molecular structure | Skeletal and cardiac sarcomere | RyR1: skeletal type | Brain, nerve, skeletal, and heart muscles |
| Giant protein (3000 kD) | RyR2: cardiac type | Homo- or hetero-tetramers | |
| Repetitive structure | 565 kD with 4 homologous subunits | One α-subunit (73 kD) | |
| Original report | Aarli et al. 1990 [7] | Mygland et al. 1992 [11] | Suzuki et al. 2005 [13] |
| Antibodies detection | ELISA (commercially available) | ELISA, Western blot | Immunoprecipitation assay |
| Myasthenia gravis titin-30 (MGT-30) near the A/I-band junction | Epitopes in both RyR1 and RyR2 | 35-S-labeled rabdomyosarcoma cell | |
| Sarcoplasmic reticulum from rabbit skeletal muscle N- and C-terminus of RyR1 sequence | Band at 70 kD | ||
| Epidemiology | 20–40% in all MG patients | 13–38% in all MG patients | 12–15% in all MG patients |
| 60–80% in MG patients older than 60 years | Mean onset age: 57 years | Mean onset age: 49 years | |
| 32% in nonthymoma MG patients older than 50 years | M : F = 1 : 1 | M : F = 1 : 1 | |
| Immunopathogenesis | T cell proliferative response to MGT-30 | Complement activation | Different from neuronal VGKC |
| Complement activation | Inhibiting Ca2+ release from sarcoplasmic reticulum | QT prolongation on electrocardiogram | |
| Myopathy in electromyogram | Autoantibodies to dihydropyridine receptor | ||
| Association with DR7 in Caucasians | or transient receptor potential canonical type-3 | ||
| Inhibiting excitation-contraction coupling | |||
| Thymoma-associated MG (T-MG) | 49–95% in T-MG | 70–80% in T-MG | 40–70% in T-MG |
| Titin epitope expression | RyR epitope expression | Kv1.4 mRNA expression | |
| Production from clonal thymic B cells | Diagnosis of thymoma | ||
| Diagnosis of thymoma (younger than 50 years) | C-terminal regions in RyR1 as epitope | ||
| Clinical presentation | Association with severe MG | More severe than anti-titin | More severe with anti-titin |
| Concomitant with myositis | Bulbar, respiratory, and neck involvement | Bulbar involvement and myasthenic crisisMyocarditis and/or myositis | |
| Myocarditis and/or myositis | Lethal arrhythmias | ||
| Treatment and management | Some late-onset MG with ocular type | Early pharmacological effect of tacrolimus | Responder to calcineurin inhibitors |
| poor prognosis in invasive thymoma | Sudden death | ||
Acknowledgments
This paper was supported by a Grant from the Japanese Ministry of Education, Science, Sports, and Culture (no. 23591255) and a Neuroimmunological Disease Research Committee Grant from the Japanese Ministry of Health, Labour, and Welfare.
References
- 1.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. The Lancet Neurology. 2009;8(5):475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams CL, Lennon VA. Thymic B lymphocyte clones from patients with myasthenia gravis secrete monoclonal striational autoantibodies reacting with myosin, α actinin, or actin. Journal of Experimental Medicine. 1986;164(4):1043–1059. doi: 10.1084/jem.164.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto T, Sato T, Sugita H. Antifilamin, antivinculin, and antitropomyosin antibodies in myasthenia gravis. Neurology. 1987;37(8):1329–1333. doi: 10.1212/wnl.37.8.1329. [DOI] [PubMed] [Google Scholar]
- 4.Ohta M, Ohta K, Itoh N, Kurobe M, Hayashi K, Nishitani H. Anti-skeletal muscle antibodies in the sera from myasthenic patients with thymoma: identification of anti-myosin, actomysin, actin, and α-actinin antibodies by a solid-phase radioimmunoassay and a Western blotting analysis. Clinica Chimica Acta. 1990;187(3):255–264. doi: 10.1016/0009-8981(90)90110-e. [DOI] [PubMed] [Google Scholar]
- 5.Aarli JA, Lefvert AK, Tonder O. Thymoma-specific antibodies in sera from patients with myasthenia gravis demonstrated by indirect haemagglutination. Journal of Neuroimmunology. 1981;1(4):421–427. doi: 10.1016/0165-5728(81)90021-7. [DOI] [PubMed] [Google Scholar]
- 6.Labeit S, Barlow DP, Gautel M, et al. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- 7.Aarli JA, Stefansson K, Marton LSG, Wollmann RL. Patients with myasthenia gravis and thymoma have in their sera IgG autoantibodies against titin. Clinical and Experimental Immunology. 1990;82(2):284–288. doi: 10.1111/j.1365-2249.1990.tb05440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romi F, Skeie GO, Gilhus NE, Aarli JA. Striational antibodies in myasthenia gravis: reactivity and possible clinical significance. Archives of Neurology. 2005;62(3):442–446. doi: 10.1001/archneur.62.3.442. [DOI] [PubMed] [Google Scholar]
- 9.Gautel M, Lakey A, Barlow DP, et al. Titin antibodies in myasthenia gravis: identification of a major immunogenic region of titin. Neurology. 1993;43(8):1581–1585. doi: 10.1212/wnl.43.8.1581. [DOI] [PubMed] [Google Scholar]
- 10.Lübke E, Freiburg A, Skeie GO, et al. Striational autoantibodies in myasthenia gravis patients recognize I- band titin epitopes. Journal of Neuroimmunology. 1998;81(1-2):98–108. doi: 10.1016/s0165-5728(97)00164-1. [DOI] [PubMed] [Google Scholar]
- 11.Mygland A, Tysnes OB, Matre R, Volpe P, Aarli JA, Gilhus NE. Ryanodine receptor autoantibodies in myasthenia gravis patients with a thymoma. Annals of Neurology. 1992;32(4):589–591. doi: 10.1002/ana.410320419. [DOI] [PubMed] [Google Scholar]
- 12.Mygland A, Tysnes OB, Matre R, Aarli JA, Gilhus NE. Anti-cardiac ryanodine receptor antibodies in thymoma-associated myasthenia gravis. Autoimmunity. 1994;17(4):327–331. doi: 10.3109/08916939409010673. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Satoh T, Yasuoka H, et al. Novel autoantibodies to a voltage-gated potassium channel KV1.4 in a severe form of myasthenia gravis. Journal of Neuroimmunology. 2005;170(1-2):141–149. doi: 10.1016/j.jneuroim.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto AM, Gajdos P, Eymard B, et al. Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Archives of Neurology. 2001;58(6):885–890. doi: 10.1001/archneur.58.6.885. [DOI] [PubMed] [Google Scholar]
- 15.Giraud M, Beaurain G, Yamamoto AM, et al. Linkage of HLA to myasthenia gravis and genetic heterogeneity depending on anti-titin antibodies. Neurology. 2001;57(9):1555–1560. doi: 10.1212/wnl.57.9.1555. [DOI] [PubMed] [Google Scholar]
- 16.Buckley C, Newsom-Davis J, Willcox N, Vincent A. Do titin and cytokine antibodies in MG patients predict thymoma or thymoma recurrence? Neurology. 2001;57(9):1579–1582. doi: 10.1212/wnl.57.9.1579. [DOI] [PubMed] [Google Scholar]
- 17.Chen XJ, Qiao J, Xiao BG, Lu CZ. The significance of titin antibodies in myasthenia gravis: correlation with thymoma and severity of myasthenia gravis. Journal of Neurology. 2004;251(8):1006–1011. doi: 10.1007/s00415-004-0479-z. [DOI] [PubMed] [Google Scholar]
- 18.Romi F, Aarli JA, Gilhus NE. Myasthenia gravis patients with ryanodine receptor antibodies have distinctive clinical features. European Journal of Neurology. 2007;14(6):617–620. doi: 10.1111/j.1468-1331.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Utsugisawa K, Nagane Y, et al. Classification of myasthenia gravis based on autoantibody status. Archives of Neurology. 2007;64(8):1121–1124. doi: 10.1001/archneur.64.8.1121. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, Utsugisawa K, Nagane Y, Satoh T, Kuwana M, Suzuki N. Clinical and immunological differences between early and late-onset myasthenia gravis in Japan. Journal of Neuroimmunology. 2011;230:148–152. doi: 10.1016/j.jneuroim.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Aarli JA. Myasthenia gravis in the elderly: is it different? Annals of the New York Academy of Sciences. 2008;1132:238–243. doi: 10.1196/annals.1405.040. [DOI] [PubMed] [Google Scholar]
- 22.Romi F, Kristoffersen EK, Aarli JA, Gilhus NE. The role of complement in myasthenia gravis: serological evidence of complement consumption in vivo. Journal of Neuroimmunology. 2005;158(1-2):191–194. doi: 10.1016/j.jneuroim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Skeie GO, Bentsen PT, Freiburg A, Aarli JA, Gilhus NE. Cell-mediated immune response against titin in myasthenia gravis: evidence for the involvement of Th1 and Th2 cells. Scandinavian Journal of Immunology. 1998;47(1):76–81. doi: 10.1046/j.1365-3083.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 24.Romi F, Skeie GO, Vedeler C, Aarli JA, Zorzato F, Gilhus NE. Complement activation by titin and ryanodine receptor autoantibodies in myasthenia gravis> A study of IgG subclasses and clinical correlations. Journal of Neuroimmunology. 2000;111(1-2):169–176. doi: 10.1016/s0165-5728(00)00394-5. [DOI] [PubMed] [Google Scholar]
- 25.Somnier FE, Skeie GO, Aarli JA, Trojaborg W. EMG evidence of myopathy and the occurrence of titin autoantibodies in patients with myasthenia gravis. European Journal of Neurology. 1999;6(5):555–563. doi: 10.1046/j.1468-1331.1999.650555.x. [DOI] [PubMed] [Google Scholar]
- 26.Skeie GO, Mygland A, Treves S, Gilhus NE, Aarli JA, Zorzato F. Ryanodine receptor antibodies in myasthenia gravis: epitope mapping and effect on calcium release in vitro. Muscle and Nerve. 2003;27(1):81–89. doi: 10.1002/mus.10294. [DOI] [PubMed] [Google Scholar]
- 27.Nakata M, Kuwabara S, Kawaguchi N, et al. Is excitation-contraction coupling impaired in myasthenia gravis? Clinical Neurophysiology. 2007;118(5):1144–1148. doi: 10.1016/j.clinph.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda E, Imai T, Hozuki T, et al. Correlation of bite force with excitation-contraction coupling time of the masseter in myasthenia gravis. Clinical Neurophysiology. 2010;121(7):1051–1058. doi: 10.1016/j.clinph.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Imai T, Tsuda E, Toyoshima T, Yoshikawa H, Motomura M, Shimohama S. Anti-ryanodine receptor-positive acetylcholine receptor-negative myasthenia gravis: evidence of impaired excitation-contraction coupling. Muscle and Nerve. 2011;43(2):294–295. doi: 10.1002/mus.21887. [DOI] [PubMed] [Google Scholar]
- 30.Takamori M. Autoantibodies against TRPC3 and ryanodine receptor in myasthenia gravis. Journal of Neuroimmunology. 2008;200(1-2):142–144. doi: 10.1016/j.jneuroim.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Maruta T, Yoshikawa H, Fukasawa S, et al. Autoantibody to dihydropyridine receptor in myasthenia gravis. Journal of Neuroimmunology. 2009;208(1-2):125–129. doi: 10.1016/j.jneuroim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Tan KM, Lennon VA, Klein CJ, Boeve BF, Pittock SJ. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70(20):1883–1890. [Google Scholar]
- 33.Lai M, Huijbers MGM, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. The Lancet Neurology. 2010;9(8):776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evoli A, Minisci C, Di Schino C, et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology. 2002;59(12):1844–1850. doi: 10.1212/01.wnl.0000032502.89361.0c. [DOI] [PubMed] [Google Scholar]
- 35.Mygland A, Kuwajima G, Mikoshiba K, Tysnes OB, Aarli JA, Gilhus NE. Thymomas express epitopes shared by the ryanodine receptor. Journal of Neuroimmunology. 1995;62(1):79–83. doi: 10.1016/0165-5728(95)00106-c. [DOI] [PubMed] [Google Scholar]
- 36.Marx A, Wilisch A, Schultz A, et al. Expression of neurofilaments and of a titin epitope in thymic epithelial tumors. Implications for the pathogenesis of Myasthenia gravis. American Journal of Pathology. 1996;148(6):1839–1850. [PMC free article] [PubMed] [Google Scholar]
- 37.Kusner LL, Mygland A, Kaminski HJ. Ryanodine receptor gene expression thymomas. Muscle and Nerve. 1998;21(10):1299–1303. doi: 10.1002/(sici)1097-4598(199810)21:10<1299::aid-mus8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Romi F, Bo L, Skeie GO, Myking A, Aarli JA, Gilhus NE. Titin and ryanodine receptor epitopes are expressed in cortical thymoma along with costimulatory molecules. Journal of Neuroimmunology. 2002;128(1-2):82–89. doi: 10.1016/s0165-5728(02)00145-5. [DOI] [PubMed] [Google Scholar]
- 39.Williams CL, Hay JE, Huiatt TW, Lennon VA. Paraneoplastic IgG striational autoantibodies produced by clonal thymic B cells and in serum of patients with myasthenia gravis and thymoma react with titin. Laboratory Investigation. 1992;66(3):331–336. [PubMed] [Google Scholar]
- 40.Takamori M, Motomura M, Kawaguchi N, et al. Anti-ryanodine receptor antibodies and FK506 in myasthenia gravis. Neurology. 2004;62(10):1894–1896. doi: 10.1212/01.wnl.0000125254.99397.68. [DOI] [PubMed] [Google Scholar]
- 41.Iwasa K, Komai K, Takamori M. Spontaneous thymoma rat as a model for myasthenic weakness caused by anti-ryanodine receptor antibodies. Muscle and Nerve. 1998;21(12):1655–1660. doi: 10.1002/(sici)1097-4598(199812)21:12<1655::aid-mus5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 42.Mygland A, Aarli JA, Matre R, Gilhus NE. Ryanodine receptor antibodies related to severity of thymoma associated myasthenia gravis. Journal of Neurology Neurosurgery and Psychiatry. 1994;57(7):843–846. doi: 10.1136/jnnp.57.7.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romi F, Gilhus NE, Varhaug JE, Myking A, Aarli JA. Disease severity and outcome in thymoma myasthenia gravis: a long-term observation study. European Journal of Neurology. 2003;10(6):701–706. doi: 10.1046/j.1468-1331.2003.00678.x. [DOI] [PubMed] [Google Scholar]
- 44.Romi F, Skeie GO, Aarli JA, Gilhus NE. The severity of myasthenia gravis correlates with the serum concentration of titin and ryanodine receptor antibodies. Archives of Neurology. 2000;57(11):1596–1600. doi: 10.1001/archneur.57.11.1596. [DOI] [PubMed] [Google Scholar]
- 45.Mygland A, Vincent A, et al. Autoantibodies in thymoma-associated myasthenia gravis with myositis or neuromyotonia . Archives of Neurology. 2000;57(4):527–531. doi: 10.1001/archneur.57.4.527. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki S, Utsugisawa K, Yoshikawa H, et al. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Archives of Neurology. 2009;66(11):1334–1338. doi: 10.1001/archneurol.2009.229. [DOI] [PubMed] [Google Scholar]
- 47.Aarli JA. Herzmyasthenie: myasthenia of the heart. Archives of Neurology. 2009;66(11):1322–1323. doi: 10.1001/archneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- 48.Sato H, Iwasaki E, Nogawa S, et al. A patient with giant cell myocarditis and myositis associated with thymoma and myasthenia gravis. Clinical Neurology. 2003;43(8):496–499. [PubMed] [Google Scholar]
- 49.Tsugawa J, Tsuboi Y, Inoue H, Suzuki S, Yamada T. Recurrent syncope due to sick sinus syndrome in a patient with myasthenia gravis associated with thymoma. Clinical Neurology. 2011;51(1):32–34. doi: 10.5692/clinicalneurol.51.32. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Katayama Y, Kusano KF, et al. Anti-KCNH2 Antibody-Induced Long QT Syndrome. Novel Acquired Form of Long QT Syndrome. Journal of the American College of Cardiology. 2007;50(18):1808–1809. doi: 10.1016/j.jacc.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 51.Nagane Y, Suzuki S, Suzuki N, Utsugisawa K. Factors associated with response to calcineurin inhibitors in myasthenia gravis. Muscle and Nerve. 2010;41(2):212–218. doi: 10.1002/mus.21462. [DOI] [PubMed] [Google Scholar]


