Figure 3. Imaging shared molecular mechanisms underlying arterial and aortic valve calcification.
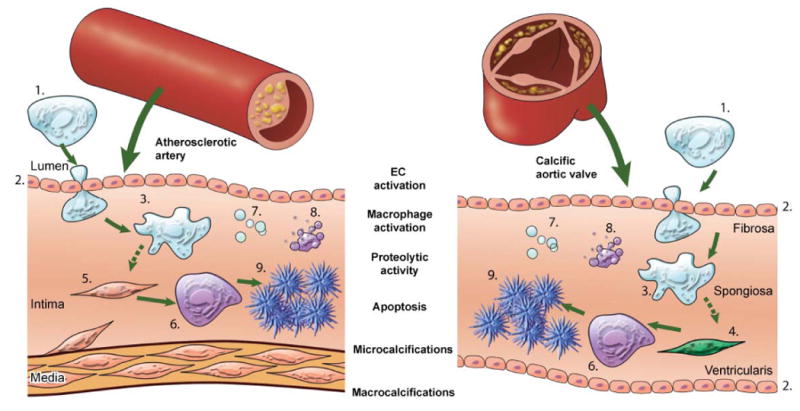
The studies cited in this review showed that calcification is an active process composed of a sequence of events, initiated by inflammation and resulting in mineralization. Pro-inflammatory monocytes (1) are recruited to a site via activated endothelial cells (EC) (2). The activation of EC causes increased expression of adhesion molecules, such as VCAM-1, which can be visualized by NIRF imaging using a VCAM-1-targeted agent. Subsequent macrophage (3) accumulation follows, which can be analyzed using NIRF macrophage-targeted nanoparticles. The release of proteolytic enzymes — including matrix metalloproteases and cathepsins — by macrophages, which stimulates the differentiation of myofibroblasts (4) and smooth muscle cells (5) into osteoblasts, can be visualized by molecular imaging using activatable imaging agents. Osteogenic activity in the form of osteoblast (6) formation and microcalcifications associated with generation of calcified matrix vesicles (7) can be identified by bisphosphonate-conjugated imaging agent, while apoptotic bodies (8) can be detected by fluorescently-tagged Annexin A5 imaging probe. Macrocalcifications (9) can be readily detected by molecular imaging and conventional imaging techniques. This schematic visualizes the theory that calcification follows similar processes in both the artery and the aortic valve, and summarizes how NIRF imaging can visualize dynamic sequences of calcification process.
