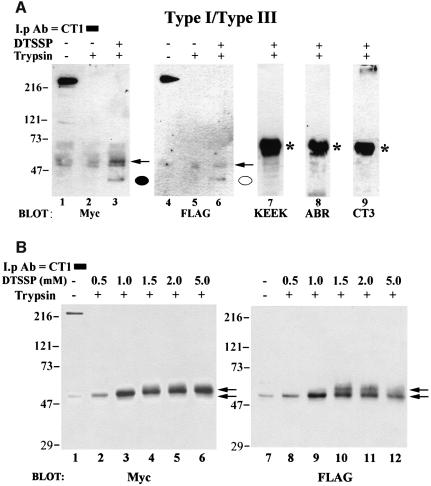
Fig. 4. Cross-linking of N- and C-termini by DTSSP. (A) Co-transfected microsomes were subjected to trypsin digestion (lanes 2, 3 and 5–9) or mock digested (lanes 1 and 4) and pelleted. Microsomes in lane 3 and lanes 6–9 were then treated with 0.5 mM DTSSP for 30 min and the reaction was terminated by the addition of 20 mM Tris pH 7.5 as described in Materials and methods. All samples were then solubilized by the addition of 1% Zwittergent, immunoprecipitated with CT1 and subjected to SDS–PAGE. Lanes 1–3 were probed with an anti-Myc antibody and the same immunoblot was stripped and re-probed with a FLAG antibody (lanes 4–6). Cross-linked N-terminal peptides co-precipitating with CT1 are designated by a closed (type I) or open (type III) circle. No DTSSP-specific bands in trypsin treated samples were observed when the same immunoblot was probed with antibodies raised against amino acids 401–414 (KEEK, lane 7) and 1883–1902 (ABR, lane 8) of the type I receptor. Similarly, no immunoreactive bands were observed when the immunoblot was probed with an antibody to the type III C-terminus (CT3, lane 9). (B) Samples were treated as in (A), except that increasing concentrations (0.5–5.0 mM) of cross-linker were used in lanes 2–6 and lanes 8–12. Increasing the DTSSP concentration was accompanied by a shift in mobility of the 42 kDa N-terminal fragment in a dose-dependent manner (indicated by arrows, see text for details). As in (A), when the blot in (B) was stripped and reprobed with KEEK, ABR or CT3 antibodies, no immunoreactive reactive bands were observed in trypsin-treated samples (data not shown). *IgG band.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.