Abstract
The popular theory six degrees of separation is used in this review as an analogy to relate all radiosensitization to oxygen. As the prime mover of all radiosensitizers, the pervasive influence of oxygen has consciously or unconsciously influenced the direction of research and development and provided the benchmark against which all other compounds and approaches are measured. It is the aim of this review to develop the six degrees of separation from oxygen analogy as a unifying framework for conceptually organizing the field and for giving context to its varied subspecializations and theories. Under such a framework, it would become possible for one area to consider questions and problems found in other areas of radiosensitization, using a common analogy, that would allow for further development and unification of this multifaceted discipline. In this review, approaches to the development of radiosensitizers and the current state of research in this field are discussed, including promising new agents in various stages of clinical development.
Introduction
The view that everything is connected to everything else according to the popular six degrees of separation theory finds corroboration on a therapeutic level in the field of radiosensitization. Analogous to a phylogenetic tree, the evolutionary lineage of radiation sensitizers from the oxygen radiosensitizers to the hypoxic cytotoxins can be traced back to a common ancestor—oxygen, the first “true” radiation sensitizer. The tree metaphor not only offers a way to keep track of the features of different radiosensitizers but also provides a framework for conceptualizing diverse mechanistic approaches. In this review, we outline the approaches to the development of radiosensitizers and discuss the current state of research.
As solid tumor growth outstrips the ability of the surrounding vasculature to supply blood and nutrients to the new cells, the tumor cell mass becomes increasingly heterogeneous, with microscopic and macroscopic areas of necrosis surrounded by cells that have very low oxygenation levels. Under normal circumstances, tumor cells would undergo apoptosis, mainly through the p53 pathway; however, these more aggressive, hypoxic cells adapt to the low oxygen levels by activation of a number of genes, including the hypoxia-inducible factor (HIF) pathway. HIF-1α, in particular, is associated with the induction of vascular endothelial growth factor (VEGF) and factors regulating glucose transport and glycolysis such as GLUT-1 and GLUT-3 [1], enabling the tumor to independently build vasculature and to support metabolic pathways. The ability of more aggressive cancer cells to survive hypoxic conditions leads to selection against apoptosis and an increased resistance to chemotherapy, as well as a propensity to metastasize. Indeed, tumor hypoxia itself [2], as well as elevated levels of HIF-1α [3], has been linked to poor treatment success characterized by drug resistance, cancer recurrence, and poor survival rates. The low oxygen levels in tumors are also associated with a poor response to radiotherapy [4]. For example, nearly 40% of breast cancers have hypoxic regions with oxygen concentrations below that required for half-maximal radiosensitivity [5].
Well-oxygenated cells show enhanced radiosensitization compared to hypoxic cells. This oxygen enhancement ratio typically ranges between 2.5 and 3.0 [6]. Oxygen enhancement ratio is described as the relative sensitivity of oxic cells/anoxic cells to the lethal effects of low linear-energy-transfer (LET) radiation: As the level of oxygen increases, so does the sensitivity of cells to radiation. Through its unique electronic configuration, oxygen, the definitive blueprint for radiosensitizers, promotes free radical formation. As the most electron-affinic cellular molecule, it is readily reduced by electrons formed from the incident radiation. On irradiation of oxygenated tumors, energy transfer results in the radiolysis of water with the initial formation of an ion radical that then forms the highly reactive hydroxyl radical after reaction with another water molecule. The presence of oxygen leads to the formation of peroxide after reaction with the hydroxyl radical. Formation of peroxide results in “fixation” or permanent cellular and DNA damage. For this reason, oxygen is the definitive hypoxic cell sensitizer and its primacy in radiation sensitization is referred to the “oxygen effect.”
In the absence of oxygen, peroxide is not formed and the sulfhydryl-containing groups such as cysteine and glutathione can restore or reconstitute DNA through hydrogen donation. As a result, hypoxia, endemic to most solid cancers, can lead to radioresistance both through increased free radical scavenging and through changes in the pattern of gene expression (e.g., induced by upregulation of the transcription factor, HIF-1α), which alters the malignant potential of tumors leading to more aggressive survival traits and resistance to radiation and chemotherapy.
The oxygen effect has driven the evolution of radiosensitization from initial attempts to increase the oxygenation of tumors with hyperbaric gases to the use of increasingly sophisticated, targeted approaches that take advantage of physiological differences in Po2 between tumors and healthy tissue.
Radiosensitizers evolved from the same common ancestor—oxygen; as a result, however different their mechanisms of action, they are interrelated through the common thread of their dependence on (or independence from) the oxygen effect. Thus, understanding the mechanism of action of a radiosensitizer amounts to understanding where on the phylogenetic tree a compound's traits exist. Mechanistic categories of radiosensitizers are described below.
Increasing Oxygen Delivery to the Tumor: Oxygen Radiosensitizers
Hyperbaric oxygen
ARCON
RSR-13
Erythropoietin
Blood transfusions
Oxyrens
Local oxygenation
The age of radiosensitization was ushered in by the pioneering work of Thomlinson and Gray [7] demonstrating that the sensitivity of tumor cells to radiation damage depended on the presence of oxygen in the tumor microenvironment. This seminal discovery, describing diffusion-limited hypoxia, paved the way for the development of radiosensitizers that attempted to increase the oxygen content of tumors. Based on this model in which oxygen delivery is compromised by limited diffusion inside tumors, early approaches to improve oxygen status of tumors involved administration of hyperbaric oxygen (HBO) [8]. However, although breathing oxygen during radiotherapy had limited success in head and neck and cervical cancers, there was no benefit in other cancers such as the CNS, bladder, and skin. Moreover, the administration of HBO has proved cumbersome and has also resulted in serious adverse effects from oxygen toxicity in clinical application. Interest in this approach has, therefore, waned significantly.
Whereas exposure to HBO and carbogen (i.e., 95% oxygen/5% carbon dioxide) increases the oxygen diffusion distance, thereby reducing chronic hypoxia, these modalities do not address acute hypoxia resulting from inadequate blood flow in tumors or anemic hypoxia which may be tumor-associated or treatment-related. The failure of HBO or carbogen as radiosensitizers may be due, therefore, to their failure to correct these other causes of tumor hypoxia.
The need to address acute, as well as chronic hypoxia, led to the investigation of ARCON (Accelerated Radiotherapy, Carbogen, and Nicotinamide) [9,10], a mixed modality approach, to attempt to overcome tumor proliferation and reverse chronic and acute hypoxia, respectively. Nicotinamide, a vasodilator, was included in the regimen to increase tumor blood flow to increase tumor Po2. However, results in squamous cell carcinoma of the head and neck were mixed, with benefit reported in laryngeal tumors but not in oral cavity and oropharyngeal tumors [10].
Other direct-line descendants of the increased tumor oxygenation approach include the use of blood transfusions and dosing of erythropoietin to increase hemoglobin concentration. However, correction of anemia with blood transfusions or erythropoietin has, in some cases, shown a decrement in patient survival and is an area of recent controversy [11,12]. An alternative approach involves increasing tumor oxygen using hemoglobin-affecting drugs dubbed Oxyrens or oxygen release enhancers. Efaproxiral (RSR-13) is a synthetic allosteric modifier of hemoglobin that decreases hemoglobin-oxygen binding capacity, thereby enabling greater oxygen delivery to tissues. However, results from ongoing trials with this drug have had limited success. In particular, although Efaproxiral was well tolerated and shown to increase oxygen delivery, the ENRICH trial in patients with breast cancer metastatic to the brain did not demonstrate a radiosensitizing effect [13], and currently, there are no ongoing, actively recruiting clinical trials with this drug. Myo-inositol trispyrophosphate or OXY111A is a novel allosteric modulator of oxygen affinity to hemoglobin that has been shown to enhance delivery of oxygen to hypoxic tissues in preclinical studies resulting in the lower expression of HIF-1α and VEGF with concomitant reduced angiogenesis compared to control [14,15]. Although no clinical or preclinical studies have described the activity of a combination of this drug with radiation, the development of OXY111A is still at an early stage with the compound currently in phase 1.
Using a different, but direct approach that is particularly applicable to nonresectable tumors, Ogawa et al. developed a radiosensitizing treatment that directly oxygenated the tumor through intratumoral administration of hydrogen peroxide either alone [16] or with a peroxidase inhibitor in the form of hyaluronic acid [17]. In a phase 1 trial, KochiOxydol-Radiation Therapy for Unresectable Carcinomas, type II (KORTUC II) was found to be well tolerated with adverse effects limited to mild local pain, with 9 of 11 patients demonstrating a complete response by RECIST criteria [18].
Oxygen Mimetics/Electron-Affinity Agents
Nitroimidazoles
Transition metal complexes
Nitric oxide
The development of “oxygen mimetics,” using the chemical properties of molecular oxygen as a template, feature compounds with high electron affinities and better diffusion properties into anoxic tissue. Unlike oxygen, which is rapidly consumed by respiring cells, these agents are less rapidly metabolized by tumors, enabling better diffusion and penetration into hypoxic regions. These compounds are described as “true radiosensitizers” in that they can theoretically substitute for oxygen in “fixing” radiation-induced damage of DNA, making it non-repairable and hence lethal. They represent the first evolutionary branch off the main trunk in the development of potential radiosensitizers.
The most well studied and representative class of oxygen mimetics is that of the nitroimidazoles, which undergo enzymatic and radiation-induced redox reactions. These agents have no intrinsic activity; their effect only becomes evident in the presence of ionizing radiation to “fix” or stabilize DNA radical lesions in an oxygen-deficient cell. In preclinical studies, the 2-nitroimidazole, misonidazole, had a higher electron affinity and was more effective than the 5-nitro imidazole, metronidazole (Flagyl), resulting in significant sensitization of tumors to radiation without concomitant effects in normal tissues [19–21]. In hypoxic tumor cells, nitroimidazoles undergo a series of enzymatic reductions, mediated by nitroreductase enzymes expressed under hypoxic conditions, leading to the generation of highly reactive anion radicals, which then irreversibly bind to cellular components.
In clinical trials, however, misonidazole caused severe peripheral and central neuropathy, which precluded safe and efficacious use in combination with radiotherapy. Notably, at clinically tolerable doses, misonidazole was ineffective as a radiosensitizer [22]. Interestingly, the less electron-affinic 5-subsituted nitroimidazole, metronidazole, did not cause the same level of neuropathy but was an inferior radiosensitizer. Although neurotoxicity associated with nitroimidazole therapy is not fully understood, this effect has been attributed to a redox reaction of catecholamine and serotonin neurotransmitters with the nitroimidazoles leading to the oxidative formation of neurotoxic semiquinone radicals [23,24]. An alternative hypothesis suggests that the generation of highly reactive nitro anion radicals is responsible for “axonal swelling with increased water retention” [25]. This alternative hypothesis was supported by data from less lipophilic analogs, including the nitrotriazole, Sanazole, that were found to be less neurotoxic [26]. Second-generation nitroimidazole radiosensitizers (e.g., nimorazole, etanidazole) were designed to increase hydrophilicity and thereby minimize exposure to neural tissues. Of these, etanidazole, had the best preclinical toxicity and efficacy profile but, unfortunately, did not afford any global benefit for patients of head and neck cancers in randomized studies [27].
Nitrogen oxides and nitric oxide, in particular, act as radiosensitizers through many different mechanisms and by direct and indirect means. Although nitric oxide as a radiosensitizer is described in more detail below, it is important to note that one mechanism of its action is in “fixing” radiation-induced damage to cellular molecules, in an analogous way to oxygen.
Transition Metal Complexes
Platinum analogs
Motexafin gadolinium
Mimicking the redox systems of the nitroimidazoles, the chemistry of transition metal complexes has been exploited for use in radiosensitization. By far, the most successful and best-known clinical example of a metal complex, which enhances the effect of radiation in hypoxic cells, is the cytotoxic drug, cisplatin. Several possible mechanisms by which platinum complexes (cisplatin, oxaliplatin, and carboplatin) sensitize tumors to radiation have been described, including inhibition of DNA repair [28]. However, like the nitroimidazoles, they also have the potential to form radicals by accepting hydrated electrons generated by ionizing radiation and “fix” radiation damage. Importantly, these agents are not selective and therefore possess significant normal tissue toxicity arising from the metal-mediated generation of the hydroxyl radical through the Fenton reaction [28].
In contrast to cisplatin, radiosensitizers based on lanthanide (III) complexes have little or no intrinsic activity but undergo futile redox cycling [29,30]: In the 3+ oxidation state, the metal complexes have high electron affinity allowing ready electron transfer between the metal and other cellular substrates such as DNA, water, oxygen, and intracellular reducing agents [31]. The subsequent catalytic transfer of the electron to oxygen not only produces reactive species like hydrogen peroxide that lead to oxidative damage by direct attack of biological macromolecules but also regenerates the initial parent compound in an unchanged form. The cycle repeats itself and can potentially deplete the cell of the reservoir of its cytoprotective agents and energy. This catalytic behavior has been termed futile redox cycling and is ultimately toxic to cells by inhibiting cellular repair [22].
Gadolinium porphyrin complexes, composed of the lanthanide coordinated to a porphyrin (motexafin gadolinium, MGd, Xcytrin or Gd(III)-tex), have been investigated as potential radiosensitizers [32–34]. In vitro studies suggest that gadolinium is able to deplete ascorbate and glutathione by direct binding, leading to a consequent increase in free radical production with improved radiosensitivity [35]. Although, preclinically, this radiosensitization effect was not uniform over different tumor types [36], clinical development demonstrated a benefit to patients [34]. In a phase 1 study, MRI showed a passive accumulation of the drug in tumor cells, presumably by extravasation from leaky vessels [32,34]. In a phase 3 study, patients with non-small cell lung cancer (NSCLC) brain metastases when treated with whole brain RT and Xcytrin experienced a significantly prolonged interval to neurological progression compared to patients treated with RT alone [37]. In 2007, Pharmacyclics received a nonapprovable letter from the US Food and Drug Administration for Xcytrin, and no further development has been reported.
Hypoxic Cytotoxins (HACs)
Mitomycin, porfiromycin, and apaziquone
Tirapazamine
AQ4N
Like a family tree, the radiosensitizer evolutionary branches diverge in an entirely different direction with hypoxic cytotoxins. These classes of compounds attempt to exploit, rather than overcome, tumor hypoxia. The drawbacks of tumor reoxygenation and the oxygen mimetics led to an interest in research on hypoxia-selective agents that had independent cytotoxic activity in addition to radiosensitization. These hypoxia-activated cytotoxins (HACs) were developed based on the hypothesis that the differences in oxygenation between normal and malignant cells can be turned from a prognostic handicap into a clinical advantage [2,38]. Conceptually, compounds that are converted to cytotoxic agents under different oxygenation states should be effective radiosensitizers. For all of these compounds, because activation occurs rapidly, a balance of key parameters was found to be critical for effective antitumor activity. Of these the reduction potential [39,40], a factor that influences the ratio of activity under hypoxic versus normoxic conditions, the relative expression of the relevant reductases in tumor versus normal tissue [41], and tumor penetration [42] have proven to be most important [43].
Mitomycin, Porfiromycin, Apaziquone
Mitomycin C (MMC) is the prototype radiosensitizing natural product cytotoxin that undergoes enzymatic reduction to generate an alkylating species; however, it has severe adverse effects attributed to its lack of preferential selectivity for hypoxic cells and, in most clinical trials, was only administered once or twice during a radiotherapy course [44]. Although it is no longer widely used clinically, MMC did demonstrate the potential of bioreductively activated drugs to selectively kill hypoxic cells in solid tumors [45]. The molecular mechanism of MMC, bioactivation through CYP 450 reductive metabolism, followed by a N-alkylation [46], represents a model for HAC. These include Porfiromycin (methyl mitomycin) [47], a close analog of MMC that has an improved therapeutic index compared to MMC. The combination of radiotherapy and Porfiromycin has been studied both preclinically [47–49] and in a number of long-term trials in patients with squamous cell cancer of the head comparing the effect of radiotherapy with either Porfiromycin or MMC [50,51]. Despite promising preclinical data, Porfiromycin was found not be as effective as MMC when combined with radiotherapy for the treatment of this tumor type [50].
Structurally similar to MMC, apaziquone (EOquin, EO9) is a bioreductive cytotoxic agent that retains the indolequinone functionality required for activity [52]. Apaziquone undergoes an oxygen dependent one electron reduction, mediated by NQO1 (NAD(P)H:Quinone oxidoreductase, EC 1.6.99.2) [53] to give a radical species with cytotoxic activity through DNA double-strand breaks. Although NQO1 is the primary activation catalyst for this drug, activity under hypoxic conditions has been reported in those tumors that have low or no levels of NQO1. Under these conditions, activation of apaziquone is catalyzed by single-electron reductases, for example, CYP450s. In addition, the drug also undergoes an oxygen-independent two electron reduction by DT diaphorase [54,55]. In early clinical studies of the drug administered IV, however, poor pharmacokinetic properties, especially rapid clearance and poor tumor penetration as well as an unfavorable toxicity profile, resulted in a reexamination of the route of delivery [56]. Apaziquone is now being developed for superficial bladder cancer and is being administered intravesically [52,57–59].
Tirapazamine
Originally identified as a herbicidal agent, the nitroxide tirapazamine (TPZ) was first described as a potential anticancer agent in 1986 [60]. The chemical structure of this compound incorporates a 1,2,4-benzotriazine-1,4 dioxide group that is susceptible to bioreduction. TPZ differs from the oxygen mimetics in that it does not “fix” radiation-induced DNA damage, but like the HAPs and HACs, is preferentially cytotoxic under hypoxic conditions. Unlike other HAPs and HACs, however, bioreduction results in the generation of a cytotoxic radical that exerts its effects directly on the surrounding tissue. In a form of futile cycling, TPZ undergoes reversible, one-electron reduction to form a neutral radical intermediate under aerobic conditions. Under hypoxic conditions, this radical is stabilized and can act as a cytotoxic agent in its own right [61]. Further hemolytic cleavage results in the expulsion of a hydroxyl radical, a known DNA-damaging species [40].
TPZ was found to markedly increase radiation-induced cell death in a dose- and schedule-dependent manner, particularly in hypoxic tumors [62]. Promising results were obtained in various preclinical studies and early-phase clinical trials in lung cancer, cervical cancer, ovarian cancer, melanoma, and head and neck cancer, with specific synergy being seen between TPZ and fractionated radiation and platinum-based chemotherapy. However, despite these encouraging results, several phase 3 trials failed to demonstrate any benefit in response rate, overall survival, or progression-free survival of adding TPZ to chemotherapy or radiation therapy in NSCLC or head and neck cancer. A recent review describes the key clinical studies in the development of TPZ [63]. Research into TPZ is now focused on the identification of the specific characteristics that resulted in the observed narrow therapeutic window and designing improved analogs. Preliminary results suggest that, once activated, TPZ reacts very quickly. A consequence of these kinetics is that cytotoxic activity occurs in a small area of cells that have the requisite level of hypoxia for activation, with little cell killing, or poor bystander effect, beyond this region. In addition, TPZ has been shown to have poor tumor penetration properties that may arise from not just poor physicochemical properties but also from vascular disrupting activity that increases tumor hypoxia and restricts tumor penetration further [64]. Analogs with improved physicochemical properties are the subject of current research [65–67]; however, the future of TPZ itself is uncertain and may depend on the ability to select those patients who have tumors that are profoundly hypoxic.
AQ4N
Exploiting the observation that N-oxides are nontoxic metabolites of tertiary amine drugs, AQ4N (banoxantrone) was designed to have antitumor activity once it was reduced from a benign, fully oxidized species, with little intrinsic activity, by CYP450 enzymes, particularly CYP3A [68–70]. The high polarity of the di-N-oxide functional groups on the side chains prevented entry into cell nuclei and DNA binding, contributing to the very low intrinsic activity of the fully oxidized derivative [69]. AQ4N was also designed to release a stable and persistent cytotoxin, AQ4 a potent DNA intercalator and topoisomerase II poison [70], similar in structure tomitoxantrone, that would, potentially, have a greater bystander effect. This feature was to circumvent the drawback of TPZ and analogs that are activated and consumed in areas of specific hypoxia. In preclinical models, AQ4N enhanced the effect of radiation in an additive manner, independent of the schedule of dosing with respect to radiation [71–73]. AQ4N has been investigated in the clinic as a single agent and in combination with radiation and temozolomide. Although single-agent trials were promising [74–76], with AQ4N exhibiting accumulation in the tumor, low overall toxicity and some partial responses in patients, results from combination trials with radiation have not been reported to date and no new studies are planned.
Direct Targeting of Hypoxia-Related Molecules
HIF-1α
Inhibitor of apoptosis (IAP) proteins, Survivin and XIAP
HIF-1α is the central transcriptional mediator of the hypoxic response in tumor cells and its overexpression is associated with increased angiogenesis, radioresistance, and a clinically poor prognosis in a variety of malignant tumors [77]. Therefore, HIF-1α and related proteins may serve as a radiotherapeutic target. Although there are no anticancer drugs that explicitly target HIF-1α, there are a surprising number of approved and experimental drugs that decrease levels of HIF-1α. These fall into a wide category of mechanisms that include antibodies against HER2, topoisomerase inhibitors, inhibitors of DNA activation through HDAC, and HSP90 inhibitors [78–80].
Several novel small molecule inhibitors of HIF-1α are now in early clinical trials. PX-478, an orally administered compound with radiosensitizing properties, derived from the clinical alkylating agent melphalan [81], is an inhibitor of HIF-1α expression [81–83]. In a phase 1 trial of PX-478 [84], 35% of patients achieved a stable disease response along with dose-proportional inhibition of HIF-1α levels. Interestingly, because PX-478 differs chemically from melphalan solely through oxidation to an N-oxide functionality, by analogy with the N-oxide AQ4N, PX-478 could also be considered as a hypoxia-activated cytotoxic agent. Nevertheless, data to date describe the activity of PX-478 as being linked to changes in HIF-1α signaling after irradiation, suggesting a mechanism independent of melphalan-like effects [85,86]. However, the association with melphalan, which does not have radiosensitizing properties, diminishes the attractiveness for the development of PX-478 in combination with radiation.
Phase 1 clinical trials with EZN-2968, an antisense oligonucleotide inhibitor of HIF-1α expression, demonstrated safety and potential activity in one patient with metastatic renal cell carcinoma [80].
The expression of HIF-1α is closely correlated with the expression of a family of proteins, the inhibitors of apoptosis (IAP) that regulate cell death [87]. Of these, survivin and XIAP [88,89] are upregulated in malignant tissue, but not in normal tissue, making them attractive therapeutic targets. Inactivation of these IAPs using antisense oligonucleotides has resulted in radiosensitization [90] as well as chemosensitization effects [91,92]. In addition, a survivin-based vaccination phase 1 study in oral cancer patients demonstrated that the peptide vaccination with survivin-based peptides was safe and had some therapeutic potential [93]. Further clinical trials, including a study in adult anaplastic glioma, are in progress [94].
Reduction of HIF-1α corresponds to a change in tumor oxygen response. Consequently, there are many compounds that are not necessarily regarded as HIF-1α inhibitors but nevertheless influence translation, expression, transcription, degradation, or clearance of HIF-1α, acting on different molecular targets. In addition to those described above, these include aminoflavone, digoxin, and hsp90 and HDAC inhibitors [80]. Although molecules that reduce HIF-1α levels have a clear potential to act as effective hypoxic radiosensitizers, data on combination therapy are mostly limited to the preclinical stage, and none of these compounds are currently being developed as radiosensitizers.
Hypoxia and/or Radiation-Activated Prodrugs of Cytotoxins (HAPs)
TH-302
PR-104
As offshoots of hypoxic cytotoxins, the hypoxia-activated prodrugs were designed to exploit tumor hypoxia, incorporating specific functional groups in their structure that can be bioreduced under hypoxia or after irradiation to release known cytotoxic agents. Unlike hypoxic cytotoxins, the hypoxia-activated prodrugs possess a significant bystander effect: These agents release a cytotoxic species with an appreciable half-life that can diffuse into tumors and exert a pronounced cell-killing effect away from the zone of activation.
For example, TH-302 [95–97], currently in phase 2 clinical trials, contains a 2-nitroimidazole functionality that serves as a hypoxic trigger releasing an achiral phosphoramidate cytotoxin, related in structure to ifosfamide. Although the alkylating nature of the TH-302 ifosfamide-like “warhead” would be expected to predominantly account for a radiosensitizing effect, it could be expected that the nitroimidazole functionality may also contribute to the effect at sufficiently elevated doses. Although preclinical studies have shown radiation sensitivity enhancement of tumor cells [98], no clinical studies of TH-302 as a potential radiosensitizer have been initiated.
Activation under deep hypoxia by nitroreductases has been exploited through the development of nitrobenzamide mustards, as exemplified by the prototypical compound, SN 23862. The nitrobenzamides exploit the metabolic switch from electron withdrawing to donating functional group interconversion; bioreduction of nitro to hydroxylamino and amino, to activate DNA cross-linking cytotoxins. Of the many nitrobenzamides studied, PR-104 is a water-soluble double prodrug that is hydrolyzed by systemic phosphatases to a lipophilic intermediate (PR-104A) that is able to diffuse to and from the hypoxic activation zone inside the tumor mass. Although hypoxic bioreduction activates a cytotoxic nitrogen mustard-alkylating agent, PR-104 can also be activated by aldo-keto reductase independent of hypoxia [99], suggesting that there would be additional cytotoxicity under normoxic conditions. In an in vitro preclinical study comparing the oxygen dependence and tissue transport properties of PR-104A with tirapazamine, performed in the context of predicting antitumor activity in combination with radiation, Hicks et al. [100] confirmed that PR-104 had different PK/PD characteristics compared to tirapazamine and was significantly more cytotoxic when combined with radiation in mouse xenograft models. These marked differences were attributed to a bystander effect resulting from diffusion of active metabolites away from severely hypoxic zones. Although a phase 1 study was completed [101], a phase 2 trial in NSCLC with docetaxel did not demonstrate sufficient efficacy for further development. In addition, the combination of PR-104 and sorafenib in hepatocellular carcinoma was not well tolerated, resulting in the termination of the trial. Current development of PR-104 is focused on AML [102].
Nitric Oxide
NO donors
TSP-1/CD47
VEGF
Is there another endogenous molecule or molecules that can not only mimic but possibly improve on the effects of oxygen as a radiosensitizer? The answer is nitric oxide (NO). Similar to the oxidative stress induced by oxygen, nitric oxide or NO can “fix” or stabilize damage to critical cellular/molecular species through nitrosative stress pathways. Studies on the role of the endogenous vasodilator, nitric oxide in cancer suggest a number of different and contradictory roles for this ubiquitous molecule, depending on its concentration, the latency of effect and the cell type. Oxidative and nitrosative stress pathways involve the generation of reactive species such as peroxynitrite (ONOO-), nitrous acid, and nitric acid that are directly and indirectly cytotoxic through mechanisms that include DNA cross-linking [103], glutathione depletion [104], protein nitrosylation [105], and inhibition of mitochondrial respiration [106].
Nitric oxide is generated endogenously by nitric oxide synthase in mammals through the oxidation of l-arginine [107]. As an uncharged free radical, nitric oxide freely diffuses across cell membranes and is able to bind to soluble guanylate cyclase (sGC), its most sensitive known target, to induce the production of cyclic GMP, thereby regulating vascular physiology.
The applicability of nitric oxide donors in a clinical oncology setting is controversial. NO at low concentrations is antiapoptotic and proangiogenic whereas, at higher levels, is proapoptotic through activation of downstream signaling pathways or after conversion to other reactive nitrogen species [108,109]. Accordingly, some studies have demonstrated improved tumor oxygenation and blood flow linked with radiosensitization resulting in tumor shrinkage, whereas other studies have reported the opposite, that is, decreased blood flow with increased rate of tumor growth, presumably linked to the expression of hypoxia-mediated transcription factors [110]. Nevertheless, more recent reports have demonstrated a radiation and chemosensitizing effect of low concentrations of nitric oxide, delivered as an NO patch or by a donor molecule [111–113].
These apparently contradictory results could also be attributed to the heterogeneous vasoresponsive capacity of tumor vessels depending on the presence or absence of smooth muscle cells and the structural relationship between vascular beds of the tumor relative to surrounding normal tissues, resulting in blood flow redistribution through steal or antisteal effects [114]. The presence of nitric oxide has also been established in conjunction with the oxygen mimetics, in that 5-nitroimidazoles [115] and sanazole [116] have been reported to release nitric oxide.
In a phase 1 study of NSCLC patients [117], tumor shrinkage and decreased blood flow was associated with administration of the nitric oxide synthase inhibitor, N-nitro-l-arginine (l-NNA) as assessed by dynamic contrast-enhanced computed tomography. The extrapolation from these data would suggest that NO donation increases tumor perfusion and therefore tumor growth. However, in a phase 2 study, prostate cancer patients who had failed primary therapy [118] were treated with low-dose sustained delivery of glyceryl trinitrate resulting in a significant decrease in prostate-specific antigen. The authors suggested that, although low-dose NO had no direct cytotoxic effect, NO decreased the emergence of a more malignant phenotype, including invasion and metastases, presumably by decreasing tumor hypoxia through improved tumor blood flow.
In contrast to directly modulating NO levels, thrombospondin 1 (TSP-1), acting through its receptor CD-47, mediates NO inhibition and antiangiogenesis. TSP-1 dysregulation has been observed in a number of human and murine tumors [119]. TSP-1 expression is frequently suppressed in tumors, preventing nitric oxide antagonism and thereby promoting NO proangiogenic effects. However, increased circulating TSP-1 levels derived from nontumorigenic stromal cells have been reported. Whereas local NO production drives tumor angiogenesis, systemic NO-mediated vasodilation preferentially enhances normal tissue perfusion at the expense of the tumor, similar to the steal effect. The known ability of endogenous TSP-1 to vasoconstrict and limit NO-driven responses in normal tissue increases tumor perfusion by decreasing circulation to healthy tissues [119]. Therapeutic concepts to modulate the TSP-1/CD47 interaction have been described in the literature [120] but have not yet advanced into formal development.
In contrast to these nonclinical experimental therapeutic modalities, bevacizumab [121], etaracizumab, sorafenib, and sunitinib are US Food and Drug Administration-approved drugs that have demonstrated clinical efficacy in oncology and act specifically, or in part, by blocking the VEGF pathway. In angiogenesis, VEGF stimulates endothelial nitric oxide release, whereas NO negatively feeds back on VEGF action [121]. This precise regulation maintains vascular homeostasis. In cancer, dysregulation occurs when VEGF, driven by tumor hypoxia, is overexpressed, leading to excessive endothelial cell proliferation and neovascularization.
Although literature describing the radiosensitizing properties of nitric oxide is extensive, nitric oxide has also been described as a radioprotectant. This seemingly contradictory behavior could [122] be attributed to NO concentration gradients with low doses resulting in cell survival signaling and high doses likely participating in the generation of free radicals, resulting in direct cytotoxicity and in the fixation of radiation-induced radical damage. Liebmann et al. [123] demonstrated that pretreatment with nitric oxide donors enhanced the survival of mice to whole body irradiation. RRx-001, a nonexplosive pernitro compound possessing a novel pharmacophore and which originated in the defense industry has been tested in animal models of radiosensitization and radioprotection. In preclinical models, preliminary data suggested that RRx-001 protected intestinal crypt cells against the effects of irradiation while significantly radiosensitizing SCCVII and RIF-1 syngeneic tumor models [124]. In a related approach, Maxhimer et al. [125] reported radioprotection of soft tissue and prevention of apoptosis in irradiated muscle in vivo by suppression of CD-47 expression, although it is possible that the mechanism could be NO-independent.
Conclusions
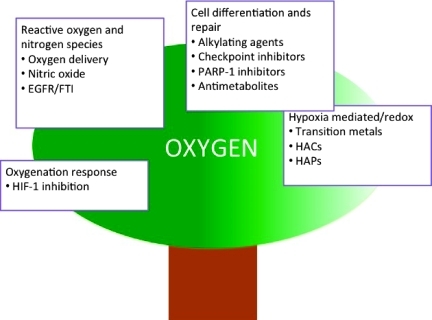
In this review, we have proposed a phylogenetic tree as a metaphor for the interrelatedness between different classes of radiosensitizers with disparate mechanisms of actions. Radiosensitization approaches, starting from molecular oxygen, as the common ancestor, initially focused on mimicking oxygen's unique properties, first by increasing intratumoral oxygen concentration and then by mimicking its electron affinity to fix the effects of radical damage (Figure 1).
Figure 1.
Radiosensitization relationship tree.
With the relative lack of success of these approaches, the development of radiosensitizers turned toward exploitation of tumor hypoxia. In this logical branching point, hypoxia became a therapeutic disadvantage that could be harnessed and used to kill tumor cells, turning the hypoxia problem into an opportunity.
Although the promise of first in class compounds like mitomycin C and misonidazole was never fully realized, subsequent generations of radiosensitizers were built on these basic concepts and became increasingly sophisticated, including chimeric compounds, that embodied different but established approaches. Today, the pipeline of potential radiosensitizers contains compounds with diverse functionalities and equally diverse sources, including the agrochemical and aerospace industry, as well as natural and biologically targeted products.
With more than 60% of cancer patients receiving radiotherapy at some period in the natural history of their diseases, there has been a recent focus on the development of molecular targeted compounds that show preclinical promise as radiosensitizers and as chemosensitizers. Before this, existing drugs with radiosensitizing properties (e.g., platinum analogs) were not widely used for their sensitizing effects, in part, because of the risk of enhancing radiation damage to normal tissues.
The six degrees of separation that we have applied to radiosensitizers to explain their connectedness to oxygen and to each other can also be used to account for their relatively neglected clinical status. Overgaard, in a recent review on radiosensitizers [126], lamented that despite intense preclinical interest, radiosensitizers were all but ignored in routine clinical practice. He attributed this clinical apathy to lack of commercial support for an area with a poor track record for the identification and development of new compounds. In a sense, the problem is that radiosensitizers are too connected: If compounds that are representative of a class of treatment repeatedly fail clinically, the perception of commercial and clinical viability of interconnected approaches is affected. For example, interest in hypoxic radiosensitizers has waned because of the continued failure of the nitroimidazoles and of tirapazamine itself. Moreover, the lack of benefit of increased O2 delivery approaches undermined not only the oxygen sensitization branch area as a whole but also because of oxygen's centrality, the entire field of radiosensitization was adversely affected. However, there remains a large unmet medical need to potentiate the effects of radiotherapy. In a similar way to exploiting the hypoxia phenomenon, the interconnectedness of radiosensitizers also represents a potential advantage, because clinical success is more likely to rejuvenate this overlapping field compared to many other therapeutic areas.
Nitric oxide represents a radical departure from other radiosensitizers. As an endogenous compound, nitric oxide may equal or surpass its molecular cousin, oxygen, as a hypoxic radiosensitizer, through pleiotropic phenotypic effects on tumor perfusion, cell signaling, mitochondrial respiration, the fixation of radiation-induced damage, and the radioprotection of normal tissue. However, unlike oxygen, in the context of radiosensitization, the clinical role and utility of NO is poorly understood, with often contradictory and controversial reported effects. Whether nitric oxide functions as a radiosensitizer may ultimately be contextual to the tumor microenvironment, depending on the architecture of the vasculature, the presence or absence of smooth muscle coverage, hypoxic status, thrombospondin 1/CD47 concentration and expression, and the effect of NO on angiogenesis. This may make NO manipulation an ideal candidate for a personalized radiosensitization approach tailored to specific patient and tumor types/microenvironmental characteristics. Effective delivery of nitric oxide both systemically and directly to the tumor may be critical to the success of this approach. Compounds that release nitric oxide or nitric oxide precursors have the potential to drive innovation and result in a new fertile branch of the radiosensitizer tree.
Using the six degrees of separation for radiosensitizers, all of which are descended from the common ancestor oxygen, it is possible to transcend the traditional boundaries of chemical structure and compound class. This way, all the compounds in the phylogenetic tree can be evaluated as a related group in the context of the oxygen effect. This knowledge can be then used in the design of new radiosensitizers and combination therapies.
References
- 1.Bel Aiba RS, Dimova EY, Görlach A, Kietzmann T. The role of hypoxia inducible factor-1 in cell metabolism—a possible target in cancer therapy. Expert Opin Ther Targets. 2006;10:583–599. doi: 10.1517/14728222.10.4.583. [DOI] [PubMed] [Google Scholar]
- 2.Boyle RG, Travers S. Hypoxia: targeting the tumour. Anticancer Agents Med Chem. 2006;6:281–286. doi: 10.2174/187152006777698169. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, Cowen RL, Stratford IJ. Hypoxia and oxidative stress. Tumour hypoxia—therapeutic considerations. Breast Cancer Res. 2001;3:328–331. doi: 10.1186/bcr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 6.Daşu A, Denekamp J. New insights into factors influencing the clinically relevant oxygen enhancement ratio. Radiother Oncol. 1998;46:269–277. doi: 10.1016/s0167-8140(97)00185-0. [DOI] [PubMed] [Google Scholar]
- 7.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer R, Hamilton-Farrell MR, van der Kleij AJ, Schmutz J, Granström G, Sicko Z, Melamed Y, Carl UM, Hartmann KA, Jansen EC, et al. Hyperbaric oxygen and radiotherapy. Strahlenther Onkol. 2005;181:113–123. doi: 10.1007/s00066-005-1277-y. [DOI] [PubMed] [Google Scholar]
- 9.Hoskin P, Rojas A, Saunders M. Accelerated radiotherapy, carbogen, and nicotinamide (ARCON) in the treatment of advanced bladder cancer: mature results of a phase II nonrandomized study. Int J Radiat Oncol Biol Phys. 2009;73:1425–1431. doi: 10.1016/j.ijrobp.2008.06.1950. [DOI] [PubMed] [Google Scholar]
- 10.Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biologybased approach in radiotherapy. Lancet Oncol. 2002;3:728–737. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- 11.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Dicato M, Plawny L. Erythropoietin in cancer patients: pros and cons. Curr Opin Oncol. 2010;22:307–311. doi: 10.1097/CCO.0b013e32833aa9de. [DOI] [PubMed] [Google Scholar]
- 13.Suh JH, Stea B, Tankel K, Marsiglia H, Belkacemi Y, Gomez H, Falcone-Lizaraso S, May J, Saunders M. Results of the phase III ENRICH (RT-016) study of efaproxiral administered concurrent with Whole Brain Radiation Therapy (WBRT) in women with brain metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:S50–S51. [Google Scholar]
- 14.Sihn G, Walter T, Klein JC, Queguiner I, Iwao H, Nicolau C, Lehn JM, Corvol P, Gasc JM. Anti-angiogenic properties of myo-inositol trispyrophosphate in ovo and growth reduction of implanted glioma. FEBS Lett. 2007;581:962–966. doi: 10.1016/j.febslet.2007.01.079. [DOI] [PubMed] [Google Scholar]
- 15.Kieda C, Greferath R, Crola da Silva C, Fylaktakidou KC, Lehn JM, Nicolau C. Suppression of hypoxia-induced HIF-1alpha and of angiogenesis in endothelial cells by myo-inositol trispyrophosphate-treated erythrocytes. Proc Natl Acad Sci USA. 2006;103:15576–15581. doi: 10.1073/pnas.0607109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa Y, Ue H, Tsuzuki K, Tadokoro M, Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et al. New radiosensitization treatment (KORTUC I) using hydrogen peroxide solution-soaked gauze bolus for unresectable and superficially exposed neoplasms. Oncol Rep. 2008;19:1389–1394. [PubMed] [Google Scholar]
- 17.Miyatake K, Kubota K, Ogawa Y, Hamada N, Murata Y, Nishioka A. Non-surgical care for locally advanced breast cancer: radiologically assessed therapeutic outcome of a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II) with systemic chemotherapy. Oncol Rep. 2010;24:1161–1168. doi: 10.3892/or_00000968. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa Y, Kubota K, Ue H, Kataoka Y, Tadokoro M, Miyatake K, Tsuzuki K, Yamanishi T, Itoh S, Hitomi J, et al. Phase I study of a new radiosensitizer containing hydrogen peroxide and sodium hyaluronate for topical tumor injection: a new enzyme-targeting radiosensitization treatment, Kochi Oxydol-Radiation Therapy for Unresectable Carcinomas, Type II (KORTUC II) Int J Oncol. 2009;34:609–618. doi: 10.3892/ijo_00000186. [DOI] [PubMed] [Google Scholar]
- 19.Brown JM. Selective radiosensitization of the hypoxic cells of mouse tumors with the nitroimidazoles metronidazole and Ro 7-0582. Radiat Res. 1975;64:633–647. [PubMed] [Google Scholar]
- 20.Dische S, Saunders MI, Lee ME, Adams GE, Flockhart IR. Clinical testing of the radiosensitizer Ro 07-0582: experience with multiple doses. Br J Cancer. 1977;35:567–579. doi: 10.1038/bjc.1977.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JM. Clinical trials of radiosensitizers: what should we expect? Int J Radiat Oncol Biol Phys. 1984;10:425–429. doi: 10.1016/0360-3016(84)90063-4. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg A, Knox S. Radiation sensitization with redox modulators: a promising approach. Int J Radiat Oncol Biol Phys. 2006;64:343–354. doi: 10.1016/j.ijrobp.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Rao DN, Mason RP. Generation of nitro radical anions of some 5-nitrofurans, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. J Biol Chem. 1987;262:11731–11736. [PubMed] [Google Scholar]
- 24.Hammami N, Drissi C, Sebai R, Araar M, Maatallah Y, Belghith L, Nagi S, Hentati F, Ben Hamouda M. Reversible metronidazole-induced encephalopathy. J Neuroradiol. 2007;34:133–136. doi: 10.1016/j.neurad.2007.01.127. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed A, Loes DJ, Bressler EL. Reversible magnetic resonance imaging findings in metronidazole-induced encephalopathy. Neurology. 1995;45:588–589. doi: 10.1212/wnl.45.3.588. [DOI] [PubMed] [Google Scholar]
- 26.Dobrowsky W, Huigol NG, Jayatilake RS, Kizilbash NI, Okkan S, Kagiya VT, Tatsuzaki H. AK-2123 (Sanazol) as a radiation sensitizer in the treatment of stage III cervical cancer: results of an IAEA multicentre randomised trial. Radiother Oncol. 2007;82:24–29. doi: 10.1016/j.radonc.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 28.Liu TZ, Lin TF, Chiu DT, Tsai KJ, Stern A. Palladium or platinum exacerbates hydroxyl radical mediated DNA damage. Free Radic Biol Med. 1997;23:155–161. doi: 10.1016/s0891-5849(96)00553-9. [DOI] [PubMed] [Google Scholar]
- 29.Shen L, Lan Z, Sun X, Shi L, Liu Q, Ni J. Proteomic analysis of lanthanum citrate-induced apoptosis in human cervical carcinoma SiHa cells. Biometals. 2010;23:1179–1189. doi: 10.1007/s10534-010-9368-3. [DOI] [PubMed] [Google Scholar]
- 30.Kostova I. Lanthanides as anticancer agents. Curr Med Chem Anticancer Agents. 2005;5:591–602. doi: 10.2174/156801105774574694. [DOI] [PubMed] [Google Scholar]
- 31.Magda D, Lepp C, Gerasimchuk N, Lee I, Sessler JL, Lin A, Biaglow JE, Miller RA. Redox cycling by motexafin gadolinium enhances cellular response to ionizing radiation by forming reactive oxygen species. Int J Radiat Oncol Biol Phys. 2001;51:1025–1036. doi: 10.1016/s0360-3016(01)01810-7. [DOI] [PubMed] [Google Scholar]
- 32.Sessler JL, Miller RA. Texaphyrins: new drugs with diverse clinical applications in radiation and photodynamic therapy. Biochem Pharmacol. 2000;59:733–739. doi: 10.1016/s0006-2952(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 33.Adis International, Limited, author. Motexafin gadolinium: gadolinium(III) texaphyrin, gadolinium texaphyrin, Gd-Tex, GdT2B2, PCI 0120. Drugs R D. 2004;5:52–57. doi: 10.2165/00126839-200405010-00012. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal DI, Nurenberg P, Becerra CR, Frenkel EP, Carbone DP, Lum BL, Miller R, Engel J, Young S, Miles D, et al. A phase I single-dose trial of gadolinium texaphyrin (Gd-Tex), a tumor selective radiation sensitizer detectable by magnetic resonance imaging. Clin Cancer Res. 1999;5:739–745. [PubMed] [Google Scholar]
- 35.Xu S, Zakian K, Thaler H, Matei C, Alfieri A, Chen Y, Koutcher JA. Effects of motexafin gadolinium on tumor metabolism and radiation sensitivity. Int J Radiat Oncol Biol Phys. 2001;49:1381–1390. doi: 10.1016/s0360-3016(00)01566-2. [DOI] [PubMed] [Google Scholar]
- 36.Bernhard EJ, Mitchell JB, Deen D, Cardell M, Rosenthal DI, Brown JM. Re-evaluating gadolinium(III) texaphyrin as a radiosensitizing agent. Cancer Res. 2000;60:86–91. [PubMed] [Google Scholar]
- 37.Mehta MP, Rodrigus P, Terhaard CH, Rao A, Suh J, Roa W, Souhami L, Bezjak A, Leibenhaut M, Komaki R, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 38.Lin AJ, Cosby LA, Shansky CW, Sartorelli AC. Potential bioreductive alkylating agents. 1. Benzoquinone derivatives. J Med Chem. 1972;15:1247–1252. doi: 10.1021/jm00282a011. [DOI] [PubMed] [Google Scholar]
- 39.Pan SS, Gonzalez H. Mitomycin antibiotic reductive potential and related pharmacological activities. Mol Pharmacol. 1990;37:966–970. [PubMed] [Google Scholar]
- 40.Lavaggi ML, Cabrera M, González M, Cerecetto H. Differential enzymatic reductions governing the differential hypoxia-selective cytotoxicities of phenazine 5,10-dioxides. Chem Res Toxicol. 2008;21:1900–1906. doi: 10.1021/tx800199v. [DOI] [PubMed] [Google Scholar]
- 41.Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R, Lewis AD. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996;88:259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- 42.Pruijn FB, Sturman JR, Liyanage HD, Hicks KO, Hay MP, Wilson WR. Extravascular transport of drugs in tumor tissue: effect of lipophilicity on diffusion of tirapazamine analogues in multicellular layer cultures. J Med Chem. 2005;48:1079–1087. doi: 10.1021/jm049549p. [DOI] [PubMed] [Google Scholar]
- 43.Siim BG, Atwell GJ, Anderson RF, Wardman P, Pullen SM, Wilson WR, Denny WA. Hypoxia-selective antitumor agents. 15. Modification of rate of nitroreduction and extent of lysosomal uptake by polysubstitution of 4-(alkylamino)-5-nitroquinoline bioreductive drugs. J Med Chem. 1997;40:1381–1390. doi: 10.1021/jm9607865. [DOI] [PubMed] [Google Scholar]
- 44.De Ridder M, Van Esch G, Engels B, Verovski V, Storme G. Hypoxic tumor cell radiosensitization: role of the iNOS/NO pathway. Bull Cancer. 2008;95:282–291. doi: 10.1684/bdc.2008.0592. [DOI] [PubMed] [Google Scholar]
- 45.Moore HW. Bioactivation as a model for drug design bioreductive alkylation. Science. 1977;197:527–532. doi: 10.1126/science.877572. [DOI] [PubMed] [Google Scholar]
- 46.Iyer VN, Szybalski W. Mitomycins and porfiromycin: chemical mechanism of activation and cross-linking of dna. Science. 1964;145:55–58. doi: 10.1126/science.145.3627.55. [DOI] [PubMed] [Google Scholar]
- 47.Keyes SR, Rockwell S, Sartorelli AC. Porfiromycin as a bioreductive alkylating agent with selective toxicity to hypoxic EMT6 tumor cells in vivo and in vitro. Cancer Res. 1985;45:3642–3645. [PubMed] [Google Scholar]
- 48.Rockwell S, Hughes CS, Keyes SR, Sartorelli AC, Kennedy KA. Porfiromycin as an adjunct to radiotherapy in young and old mice. Exp Gerontol. 1993;28:281–293. doi: 10.1016/0531-5565(93)90035-c. [DOI] [PubMed] [Google Scholar]
- 49.Rockwell S, Keyes SR, Sartorelli AC. Preclinical studies of porfiromycin as an adjunct to radiotherapy. Radiat Res. 1988;116:100–113. [PubMed] [Google Scholar]
- 50.Haffty BG, Wilson LD, Son YH, Cho EI, Papac RJ, Fischer DB, Rockwell S, Sartorelli AC, Ross DA, Sasaki CT, et al. Concurrent chemo-radiotherapy with mitomycin C compared with porfiromycin in squamous cell cancer of the head and neck: final results of a randomized clinical trial. Int J Radiat Oncol Biol Phys. 2005;61:119–128. doi: 10.1016/j.ijrobp.2004.07.730. [DOI] [PubMed] [Google Scholar]
- 51.Haffty BG, Son YH, Wilson LD, Papac R, Fischer D, Rockwell S, Sartorelli AC, Ross D, Sasaki CT, Fischer JJ. Bioreductive alkylating agent porfiromycin in combination with radiation therapy for the management of squamous cell carcinoma of the head and neck. Radiat Oncol Investig. 1997;5:235–245. doi: 10.1002/(SICI)1520-6823(1997)5:5<235::AID-ROI4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Witjes JA, Kolli PS. Apaziquone for non-muscle invasive bladder cancer: a critical review. Expert Opin Investig Drugs. 2008;17:1085–1096. doi: 10.1517/13543784.17.7.1085. [DOI] [PubMed] [Google Scholar]
- 53.Workman P. Enzyme-directed bioreductive drug development revisited: a commentary on recent progress and future prospects with emphasis on quinone anticancer agents and quinone metabolizing enzymes, particularly DT-diaphorase. Oncol Res. 1994;6:461–475. [PubMed] [Google Scholar]
- 54.Walton MI, Sugget N, Workman P. The role of human and rodent DT-diaphorase in the reductive metabolism of hypoxic cell cytotoxins. Int J Radiat Oncol Biol Phys. 1992;22:643–647. doi: 10.1016/0360-3016(92)90495-4. [DOI] [PubMed] [Google Scholar]
- 55.Walton MI, Bibby MC, Double JA, Plumb JA, Workman P. DT-diaphorase activity correlates with sensitivity to the indoloquinone EO9 in mouse and human colon carcinomas. Eur J Cancer. 1992;28A:1597–1600. doi: 10.1016/0959-8049(92)90049-8. [DOI] [PubMed] [Google Scholar]
- 56.Schellens JH, Planting AS, van Acker BA, Loos WJ, de Boer-Dennert M, van der Burg ME, Koier I, Krediet RT, Stoter G, Verweij J. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994;86:906–912. doi: 10.1093/jnci/86.12.906. [DOI] [PubMed] [Google Scholar]
- 57.Jain A, Phillips RM, Scally AJ, Lenaz G, Beer M, Puri R. Response of multiple recurrent TaT1 bladder cancer to intravesical apaziquone (EO9): comparative analysis of tumor recurrence rates. Urology. 2009;73:1083–1086. doi: 10.1016/j.urology.2007.12.062. [DOI] [PubMed] [Google Scholar]
- 58.Hendricksen K, van der Heijden AG, Cornel EB, Vergunst H, de Reijke TM, van Boven E, Smits GA, Puri R, Gruijs S, Witjes JA. Two-year follow-up of the phase II marker lesion study of intravesical apaziquone for patients with non-muscle invasive bladder cancer. World J Urol. 2009;27:337–342. doi: 10.1007/s00345-009-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spectrum Pharma. [June 19, 2011]. Available at http://www.spectrumpharma.com/eoquin.html.
- 60.Zeman EM, Brown JM, Lemmon MJ, Hirst VK, Lee WW. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986;12:1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- 61.Brown JM. SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer. 1993;67:1163–1170. doi: 10.1038/bjc.1993.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown JM, Lemmon MJ. Tumor hypoxia can be exploited to preferentially sensitize tumors to fractionated irradiation. Int J Radiat Oncol Biol Phys. 1991;20:457–461. doi: 10.1016/0360-3016(91)90057-b. [DOI] [PubMed] [Google Scholar]
- 63.Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs. 2009;18:77–87. doi: 10.1517/13543780802567250. [DOI] [PubMed] [Google Scholar]
- 64.Huxham LA, Kyle AH, Baker JH, McNicol KL, Minchinton AI. Exploring vascular dysfunction caused by tirapazamine. Microvasc Res. 2008;75:247–255. doi: 10.1016/j.mvr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Hay MP, Hicks KO, Pchalek K, Lee HH, Blaser A, Pruijn FB, Anderson RF, Shinde SS, Wilson WR, Denny WA. Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins. J Med Chem. 2008;51:6853–6865. doi: 10.1021/jm800967h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hicks KO, Siim BG, Jaiswal JK, Pruijn FB, Fraser AM, Patel R, Hogg A, Liyanage HD, Dorie MJ, Brown JM, et al. Pharmacokinetic/pharmacodynamic modeling identifies SN30000 and SN29751 as tirapazamine analogues with improved tissue penetration and hypoxic cell killing in tumors. Clin Cancer Res. 2010;16:4946–4957. doi: 10.1158/1078-0432.CCR-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hicks KO, Pruijn FB, Secomb TW, Hay MP, Hsu R, Brown JM, Denny WA, Dewhirst MW, Wilson WR. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxiatargeted anticancer drugs. J Natl Cancer Inst. 2006;98:1118–1128. doi: 10.1093/jnci/djj306. [DOI] [PubMed] [Google Scholar]
- 68.Nishida CR, Ortiz de Montellano PR. Reductive heme-dependent activation of the n-oxide prodrug AQ4N by nitric oxide synthase. J Med Chem. 2008;51:5118–5120. doi: 10.1021/jm800496s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patterson LH, McKeown SR. AQ4N: a new approach to hypoxiaactivated cancer chemotherapy. Br J Cancer. 2000;83:1589–1593. doi: 10.1054/bjoc.2000.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patterson LH, McKeown SR, Ruparelia K, Double JA, Bibby MC, Cole S, Stratford IJ. Enhancement of chemotherapy and radiotherapy of murine tumours by AQ4N, a bioreductively activated anti-tumour agent. Br J Cancer. 2000;82:1984–1990. doi: 10.1054/bjoc.2000.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hejmadi MV, McKeown SR, Friery OP, McIntyre IA, Patterson LH, Hirst DG. DNA damage following combination of radiation with the bioreductive drug AQ4N: possible selective toxicity to oxic and hypoxic tumour cells. Br J Cancer. 1996;73:499–505. doi: 10.1038/bjc.1996.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKeown SR, Friery OP, McIntyre IA, Hejmadi MV, Patterson LH, Hirst DG. Evidence for a therapeutic gain when AQ4N or tirapazamine is combined with radiation. Br J Cancer Suppl. 1996;27:S39–S42. [PMC free article] [PubMed] [Google Scholar]
- 73.McKeown SR, Hejmadi MV, McIntyre IA, McAleer JJ, Patterson LH. AQ4N: an alkylaminoanthraquinone N-oxide showing bioreductive potential and positive interaction with radiation in vivo. Br J Cancer. 1995;72:76–81. doi: 10.1038/bjc.1995.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papadopoulos KP, Goel S, Beeram M, Wong A, Desai K, Haigentz M, Milián ML, Mani S, Tolcher A, Lalani AS, et al. A phase 1 open-label, accelerated doseescalation study of the hypoxia-activated prodrug AQ4N in patients with advanced malignancies. Clin Cancer Res. 2008;14:7110–7115. doi: 10.1158/1078-0432.CCR-08-0483. [DOI] [PubMed] [Google Scholar]
- 75.Albertella MR, Loadman PM, Jones PH, Phillips RM, Rampling R, Burnet N, Alcock C, Anthoney A, Vjaters E, Dunk CR, et al. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res. 2008;14:1096–1104. doi: 10.1158/1078-0432.CCR-07-4020. [DOI] [PubMed] [Google Scholar]
- 76.Sarantopoulos JJ, Tolcher AW, Wong A, Goel S, Beeram M, Lam G, Desai K, Woody K, Mani S, Papadopoulos KP. Banoxantrone (AQ4N), tissue CYP 450 targeted prodrug: the results of a phase I study using an accelerated dose escalation. J Clin Oncol. 2006;24:2011. [Google Scholar]
- 77.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Semenza GL. Development of novel therapeutic strategies that target HIF-1. Expert Opin Ther Targets. 2006;10:267–280. doi: 10.1517/14728222.10.2.267. [DOI] [PubMed] [Google Scholar]
- 79.Koh MY, Spivak-Kroizman TR, Powis G. Inhibiting the hypoxia response for cancer therapy: the new kid on the block. Clin Cancer Res. 2009;15:5945–5946. doi: 10.1158/1078-0432.CCR-09-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–2786. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palayoor ST, Mitchell JB, Cerna D, Degraff W, John-Aryankalayil M, Coleman CN. PX-478, an inhibitor of hypoxia-inducible factor-1alpha, enhances radiosensitivity of prostate carcinoma cells. Int J Cancer. 2008;123:2430–2437. doi: 10.1002/ijc.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jacoby JJ, Erez B, Korshunova MV, Williams RR, Furutani K, Takahashi O, Kirkpatrick L, Lippman SM, Powis G, O'Reilly MS, et al. Treatment with HIF-1alpha antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J Thorac Oncol. 2010;5:940–949. doi: 10.1097/JTO.0b013e3181dc211f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, Powis G. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 84.Tibes R, Falchook GS, Von Hoff DD, Weiss GJ, Iyengar T, Kurzrock R, Pestano L, Lowe AM, Herbst RS. Results from a phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1a. J Clin Oncol. 2010;28(suppl):3076. [Google Scholar]
- 85.Schwartz DL, Bankson JA, Lemos R, Jr, Lai SY, Thittai AK, He Y, Hostetter G, Demeure MJ, Von Hoff DD, Powis G. Radiosensitization and stromal imaging response correlates for the HIF-1 inhibitor PX-478 given with or without chemotherapy in pancreatic cancer. Mol Cancer Ther. 2010;9:2057–2067. doi: 10.1158/1535-7163.MCT-09-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz DL, Powis G, Thitai-Kumar A, He Y, Bankson J, Williams R, Lemos R, Oh J, Volgin A, Soghomonyan S, et al. The selective hypoxia inducible factor-1 inhibitor PX-478 provides in vivo radiosensitization through tumor stromal effects. Mol Cancer Ther. 2009;8:947–958. doi: 10.1158/1535-7163.MCT-08-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 88.Wei H, Wang C, Chen L. Proliferating cell nuclear antigen, survivin, and CD34 expressions in pancreatic cancer and their correlation with hypoxia-inducible factor 1alpha. Pancreas. 2006;32:159–163. doi: 10.1097/01.mpa.0000202961.71600.9b. [DOI] [PubMed] [Google Scholar]
- 89.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 90.Cao C, Mu Y, Hallahan DE, Lu B. XIAP and survivin as therapeutic targets for radiation sensitization in preclinical models of lung cancer. Oncogene. 2004;23:7047–7052. doi: 10.1038/sj.onc.1207929. [DOI] [PubMed] [Google Scholar]
- 91.Hu Y, Cherton-Horvat G, Dragowska V, Baird S, Korneluk RG, Durkin JP, Mayer LD, LaCasse EC. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 92.Olie RA, Simões-Wüst AP, Baumann B, Leech SH, Fabbro D, Stahel RA, Zangemeister-Wittke U. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 93.Miyazaki A, Kobayashi J, Torigoe T, Hirohashi Y, Yamamoto T, Yamaguchi A, Asanuma H, Takahashi A, Michifuri Y, Nakamori K, et al. Phase I clinical trial of survivin-derived peptide vaccine therapy for patients with advanced or recurrent oral cancer. Cancer Sci. 2011;102:324–329. doi: 10.1111/j.1349-7006.2010.01789.x. [DOI] [PubMed] [Google Scholar]
- 94.Survivin vaccine therapy for patients with malignant gliomas. [June 19, 2011]. Available at: clinicaltrialsfeeds.org/clinical-trials/show/NCT01250470.
- 95.Duan JX, Jiao H, Kaizerman J, Stanton T, Evans JW, Lan L, Lorente G, Banica M, Jung D, Wang J, et al. Potent and highly selective hypoxia-activated achiral phosphoramidate mustards as anticancer drugs. J Med Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 96.Hu J, Handisides DR, Van Valckenborgh E, De Raeve H, Menu E, Vande Broek I, Liu Q, Sun JD, Van Camp B, Hart CP, et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood. 2010;116:1524–1527. doi: 10.1182/blood-2010-02-269126. [DOI] [PubMed] [Google Scholar]
- 97.Li S, Zhang J, Li J, Chen D, Matteucci M, Curd J, Duan JX. Inhibition of both thioredoxin reductase and glutathione reductase may contribute to the anticancer mechanism of TH-302. Biol Trace Elem Res. 2010;136:294–301. doi: 10.1007/s12011-009-8544-1. [DOI] [PubMed] [Google Scholar]
- 98.Bischoff P, Altmeyer A, Dumont F. Radiosensitising agents for the radiotherapy of cancer: advances in traditional and hypoxia targeted radiosensitisers. Expert Opin Ther Pat. 2009;19:643–662. doi: 10.1517/13543770902824172. [DOI] [PubMed] [Google Scholar]
- 99.Guise CP, Abbattista MR, Singleton RS, Holford SD, Connolly J, Dachs GU, Fox SB, Pollock R, Harvey J, Guilford P, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70:1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]
- 100.Hicks KO, Myint H, Patterson AV, Pruijn FB, Siim BG, Patel K, Wilson WR. Oxygen dependence and extravascular transport of hypoxia-activated prodrugs: comparison of the dinitrobenzamide mustard PR-104A and tirapazamine. Int J Radiat Oncol Biol Phys. 2007;69:560–571. doi: 10.1016/j.ijrobp.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 101.Jameson MB, Rischin D, Pegram M, Gutheil J, Patterson AV, Denny WA, Wilson WR. A phase I trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65:791–801. doi: 10.1007/s00280-009-1188-1. [DOI] [PubMed] [Google Scholar]
- 102.Proacta Updates PR1-4 Phase II Program, Vol. 2010. [June 19, 2011]. Available at: http://www.proacta.com/news/pr_feb0110.htm.
- 103.Edfeldt NB, Harwood EA, Sigurdsson ST, Hopkins PB, Reid BR. Solution structure of a nitrous acid induced DNA interstrand cross-link. Nucleic Acids Res. 2004;32:2785–2794. doi: 10.1093/nar/gkh606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bonavida B, Khineche S, Huerta-Yepez S, Garbán H. Therapeutic potential of nitric oxide in cancer. Drug Resist Updat. 2006;9:157–173. doi: 10.1016/j.drup.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Nelson EJ, Connolly J, McArthur P. Nitric oxide and S-nitrosylation: excitotoxic and cell signaling mechanism. Biol Cell. 2003;95:3–8. doi: 10.1016/s0248-4900(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 106.Meffert MK, Premack BA, Schulman H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron. 1994;12:1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 107.Tayeh MA, Marletta MA. Macrophage oxidation of l-arginine to nitric oxide, nitrite, and nitrate. Tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. [PubMed] [Google Scholar]
- 108.Brüne B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10:864–869. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- 109.Bonavida B, Baritaki S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: downregulation of the NF-κB/Snail/YY1/RKIP circuitry. Nitric Oxide. 2011;24:1–7. doi: 10.1016/j.niox.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Shan SQ, Rosner GL, Braun RD, Hahn J, Pearce C, Dewhirst MW. Effects of diethylamine/nitric oxide on blood perfusion and oxygenation in the R3230Ac mammary carcinoma. Br J Cancer. 1997;76:429–437. doi: 10.1038/bjc.1997.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frederiksen LJ, Sullivan R, Maxwell LR, Macdonald-Goodfellow SK, Adams MA, Bennett BM, Siemens DR, Graham CH. Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res. 2007;13:2199–2206. doi: 10.1158/1078-0432.CCR-06-1807. [DOI] [PubMed] [Google Scholar]
- 112.Bonavida B, Baritaki S, Huerta-Yepez S, Vega MI, Chatterjee D, Yeung K. Novel therapeutic applications of nitric oxide donors in cancer: roles in chemo- and immunosensitization to apoptosis and inhibition of metastases. Nitric Oxide. 2008;19:152–157. doi: 10.1016/j.niox.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 113.Gao X, Saha D, Kapur P, Anthony T, Livingston EH, Huerta S. Radiosensitization of HT-29 cells and xenografts by the nitric oxide donor DETANONOate. J Surg Oncol. 2009;100:149–158. doi: 10.1002/jso.21318. [DOI] [PubMed] [Google Scholar]
- 114.Jordan BF, Misson P, Demeure R, Baudelet C, Beghein N, Gallez B. Changes in tumor oxygenation/perfusion induced by the no donor, isosorbide dinitrate, in comparison with carbogen: monitoring by EPR and MRI. Int J Radiat Oncol Biol Phys. 2000;48:565–570. doi: 10.1016/s0360-3016(00)00694-5. [DOI] [PubMed] [Google Scholar]
- 115.Girard M, Clairmont F, Maneckjee A, Mousseau N, Dawson B, Whitehouse L. 5-Nitroimidazoles. II: Unexpected reactivity of ronidazole and dimetridazole with thiols. Can J Chem. 1993;71:1349–1352. [Google Scholar]
- 116.Kondakova IV, Tcheredova VV, Zagrebelnaya GV, Cherdyntseva NV, Kagiya TV, Choinzonov EL. Production of nitric oxide by hypoxic radiosensitizer sanazole. Exp Oncol. 2004;26:329–333. [PubMed] [Google Scholar]
- 117.Ng QS, Goh V, Milner J, Stratford MR, Folkes LK, Tozer GM, Saunders MI, Hoskin PJ. Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: a phase I study. Lancet Oncol. 2007;8:111–118. doi: 10.1016/S1470-2045(07)70001-3. [DOI] [PubMed] [Google Scholar]
- 118.Siemens DR, Heaton JP, Adams MA, Kawakami J, Graham CH. Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology. 2009;74:878–883. doi: 10.1016/j.urology.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for antiangiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100:712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 121.Libert N, Tourtier JP, Védrine L, Chargari C. Inhibitors of angiogenesis: new hopes for oncologists, new challenges for anesthesiologists. Anesthesiology. 2010;113:704–712. doi: 10.1097/ALN.0b013e3181ed098d. [DOI] [PubMed] [Google Scholar]
- 122.Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, Tsokos M, Wink DA, Roberts DD. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liebmann J, DeLuca AM, Coffin D, Keefer LK, Venzon D, Wink DA, Mitchell JB. In vivo radiation protection by nitric oxide modulation. Cancer Res. 1994;54:3365–3368. [PubMed] [Google Scholar]
- 124.Ning S, Bednarski M, Oronsky B, Scicinski J, Saul G, Knox S. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research, Orlando, Florida, 2–6 April 2011. Philadelphia (PA): AACR; 2011. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials [abstract] Abstract 676. [DOI] [PubMed] [Google Scholar]
- 125.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]