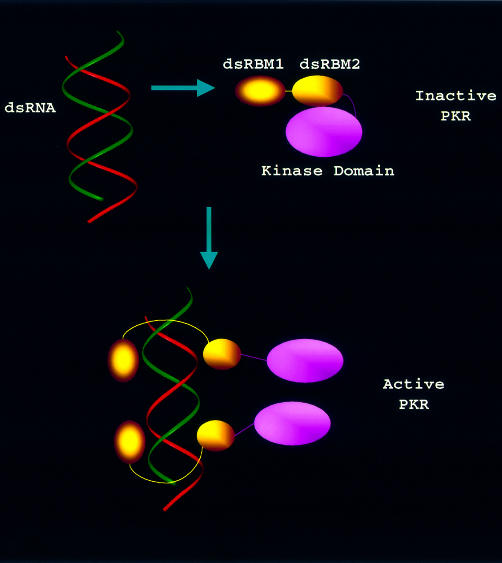
Fig. 6. A model for dsRNA-mediated PKR activation. In the inactive PKR, dsRBM2 contacts with the kinase domain for a ‘closed’ conformation whereas dsRBM1 is unconstrained, fluctuating with millisecond motions. Viral dsRNA is first anchored to the free dsRBM1, inducing the cooperative binding to dsRBM2, which then exposes the kinase domain. This would also relax the major dimerization region (244–296), which is otherwise restrained due to the interaction between dsRBM2 and the kinase domain. Such concerted conformational change then results in PKR dimerization, autophosphorylation and activation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.