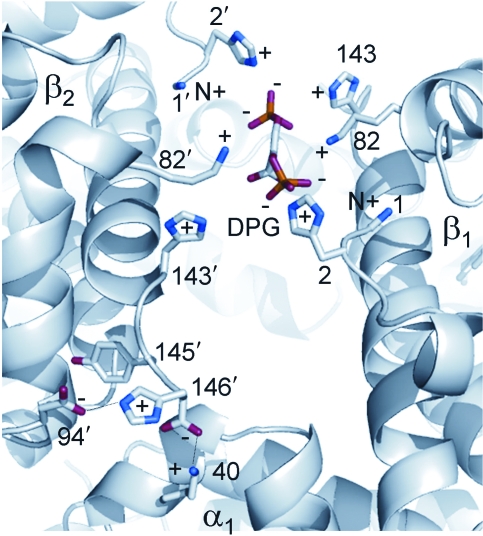
FIG. 5.
Binding of 2,3-DPG in the central cavity between the β1 and β2 chains of deoxyHb. Also shown (lower-left corner) is an intrachain salt bridge that is formed in deoxyHb between the imidazole ring of the N-terminal β146(HC3)His and the negatively charged β94(FG1)Asp. This bond increases the affinity of FG1 Asp for protons and therefore contributes to the Bohr effect. The protons are released upon transition to R-state oxyHb.