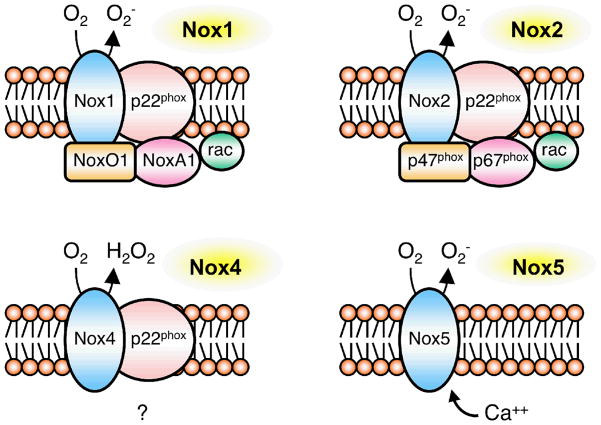
Figure 3.
Schematic representation of components of the vascular NADPH oxidases (based on Refs 2, 55–59). Nox1 is activated by homologs ofp47phox and p67phox – Nox-organizer 1 (NoxO1) and Nox-activator 1 (NoxA1). Nox2 requires recruitment ofp47phox and p67phox. Nox4 does not appear to require any cytosolic subunits, and Nox5 is activated by Ca2+. Nox1, Nox2 and Nox4 require association with p22phox to function normally. Nox4 might produce H2O2 directly (58). The subcellular localization of Nox isoforms and the site of superoxide generation varies with cell type and presumably function (2). While some isoforms are expressed in the cell membrane, additional sites of expression include the nucleus (or perinuclear) and the endoplasmic reticulum.