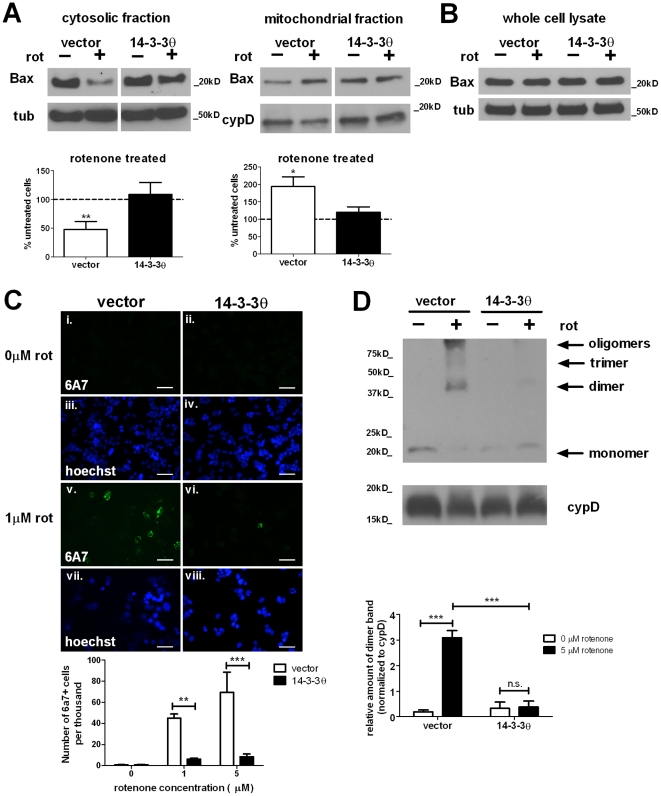
Figure 2. Rotenone-induced Bax activation is reduced in 14-3-3θ-overexpressing cells.
a) Less Bax translocated to mitochondria in 14-3-3θ cells in response to rotenone. After treatment with 5 µM rotenone for 24 hours, vector control and 14-3-3θ cell lysates were subfractionated into cytosolic and mitochondrial fractions and immunoblotted with a polyclonal rabbit antibody against Bax. For each fraction, lanes for vector control and 14-3-3θ cells are from the same gel and exposure time but are separated for clarity with regard to quantification. Bax levels were normalized to tubulin for the cytosolic fraction or cyclophilin D for the mitochondrial fraction. Bax levels for rotenone-treated cells are shown as the relative percentage of the corresponding untreated cells. Densitometric quantification included seven separate experiments. Error bars reflect SEM. *p<0.05, **p<0.01 (one sample t-test). b) Total Bax levels were unchanged with rotenone treatment in either cell line. After treatment with 5 µM rotenone for 24 hours, whole cell lysates were immunoblotted with an anti-Bax antibody. c) Fewer 14-3-3θ cells were positive for activated Bax upon rotenone treatment. After treatment without (i-iv) or with rotenone (v-viii) for 16 hours, vector control and 14-3-3θ cells were fixed in 2% paraformaldehyde and immunostained with a monoclonal mouse antibody against the active Bax conformation (6A7) and a goat Alexa 488-conjugated anti-mouse secondary antibody (i, ii, v, vi). Nuclei were stained with Hoechst 33342 (iii, iv, vii, viii). The number of 6A7-positive cells was quantitated with rater blind to experimental conditions. Error bars reflect SEM. **p<0.01, ***p<0.001 (Bonferroni's multiple comparison test). Scale bar = 50 µm. d) Rotenone-induced Bax oligomerization was reduced in 14-3-3θ cells. Vector control and 14-3-3θ stable cells were treated with 5 µM rotenone for 24 hours. Mitochondrially-enriched fractions were crosslinked and immunoblotted for oligomers with an anti-Bax antibody. Cyclophilin D served as loading control. Densitometric quantification includes three independent experiments. Error bars reflect SEM. ***p<0.001 (Bonferroni's multiple comparison test). n.s. = non-significant.